Themed collection Most popular 2021 organic chemistry articles, 2021

Boronic acid based dynamic click chemistry: recent advances and emergent applications
Fundamental progress, current developments, and rapidly growing applications of iminoboronate and salicylhydroxamic–boronate conjugate esters are deliberated.
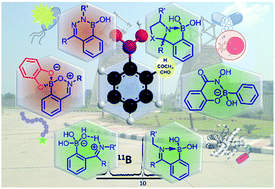
Chem. Sci., 2021,12, 1585-1599
https://doi.org/10.1039/D0SC05009A
Water as the reaction medium in organic chemistry: from our worst enemy to our best friend
A review that highlights water as the logical reaction medium in which organic chemistry can be practiced. The key roles that water can play in directing reaction outcomes, including impacting mechanistic features, are discussed using selected examples.

Chem. Sci., 2021,12, 4237-4266
https://doi.org/10.1039/D0SC06000C
Hexafluoroisopropanol: the magical solvent for Pd-catalyzed C–H activation
Among numerous solvents available for chemical transformations, 1,1,1,3,3,3-hexafluoro-2-propanol (popularly known as HFIP) has attracted enough attention of the scientific community in recent years.
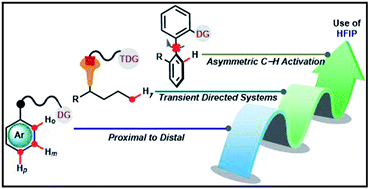
Chem. Sci., 2021,12, 3857-3870
https://doi.org/10.1039/D0SC06937J
Recent development in transition metal-catalysed C–H olefination
Transition metal-catalysed functionalizations of inert C–H bonds to construct C–C bonds represent an ideal route in the synthesis of valuable organic molecules.
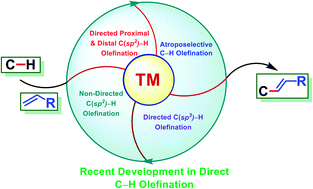
Chem. Sci., 2021,12, 2735-2759
https://doi.org/10.1039/D0SC05555G
Solvent coordination to palladium can invert the selectivity of oxidative addition
In the presence of the bulky monophosphine PtBu3, palladium usually prefers to react with Ar–Cl over Ar–OTf bonds. However, strongly coordinating solvents can bind to palladium, inducing a reversal of selectivity.

Chem. Sci., 2022,13, 1618-1628
https://doi.org/10.1039/D1SC05862B
Cracking the immune fingerprint of metal–organic frameworks
Unveiling the immune fingerprint of MOFs: the design of customized immune-active MOF nanoplatforms for targeting specific diseases will open new avenues for their biomedical applications.

Chem. Sci., 2022,13, 934-944
https://doi.org/10.1039/D1SC04112F
Galbofloxacin: a xenometal-antibiotic with potent in vitro and in vivo efficacy against S. aureus
Galbofloxacin, a novel theranostic xenosiderophore antibiotic, exhibits unparalleled potency in combating S. aureus infections in vivo.
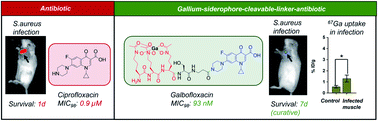
Chem. Sci., 2021,12, 14546-14556
https://doi.org/10.1039/D1SC04283A
Enantioselective palladaelectro-catalyzed C–H olefinations and allylations for N–C axial chirality
Enantioselective palladaelectro-catalyzed C–H alkenylations and allylations were achieved by the means of an easily-accessible amino acid for the synthesis of N–C axially chiral indole biaryls.
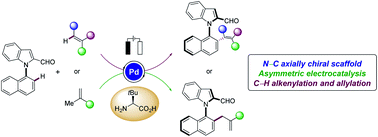
Chem. Sci., 2021,12, 14182-14188
https://doi.org/10.1039/D1SC04687J
Origin of enantioselectivity reversal in Lewis acid-catalysed Michael additions relying on the same chiral source
Enantiodivergence is an important concept in asymmetric catalysis that enables access to both enantiomers of a product relying on the same chiral source as reagent.
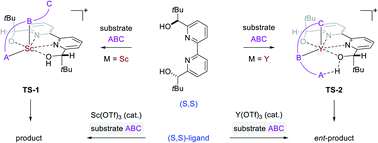
Chem. Sci., 2021,12, 14133-14142
https://doi.org/10.1039/D1SC03741B
Femtosecond stimulated Raman spectroscopy – guided library mining leads to efficient singlet fission in rubrene derivatives
In the race to find efficient singlet fission materials, picking a winner is not easy. Femtosecond stimulated Raman spectroscopy can help us choose the best candidates, as demonstrated here in choosing from a library of rubrene derivatives.
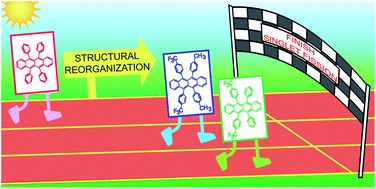
Chem. Sci., 2021,12, 13825-13835
https://doi.org/10.1039/D1SC04251C
New Cy5 photosensitizers for cancer phototherapy: a low singlet–triplet gap provides high quantum yield of singlet oxygen
The electron-withdrawing group at the meso-position of Thio-Cy5 could dramatically reduce the singlet–triplet energy gap, and speed up the intersystem crossing process.
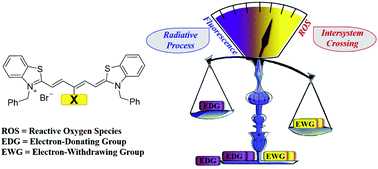
Chem. Sci., 2021,12, 13809-13816
https://doi.org/10.1039/D1SC04570A
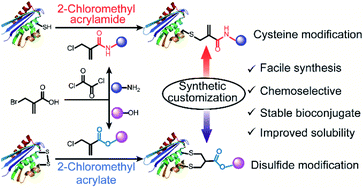
Chemoselective cysteine or disulfide modification via single atom substitution in chloromethyl acryl reagents
2-Chloromethyl acryl derivatives (acrylamides and acrylates) can serve as simple and versatile bioconjugation reagents to achieve site-selective cysteine and disulfide modification on demand and with high efficiency.
Chem. Sci., 2021,12, 13321-13330
https://doi.org/10.1039/D1SC03250J
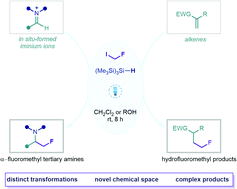
Visible light-mediated radical fluoromethylation via halogen atom transfer activation of fluoroiodomethane
Generation of fluoromethyl radicals via visible light-mediated halogen atom transfer activation of fluoroiodomethane facilitates both the multicomponent synthesis of α-fluoromethyl amines and the hydrofluoromethylation of electron-deficient alkenes.
Chem. Sci., 2021,12, 12812-12818
https://doi.org/10.1039/D1SC04554G
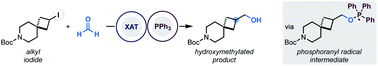
Radical hydroxymethylation of alkyl iodides using formaldehyde as a C1 synthon
Halogen-atom transfer (XAT) based on phosphoranyl radical chemistry enables the hydroxymethylation of alkyl iodides with formaldehyde.
Chem. Sci., 2021,12, 10448-10454
https://doi.org/10.1039/D1SC03083C
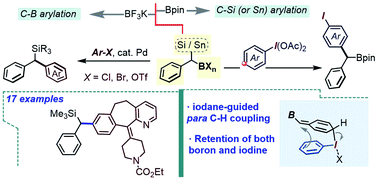
Exploring benzylic gem-C(sp3)–boron–silicon and boron–tin centers as a synthetic platform
This work explores divergent reactivity of the benzylic gem-boron–silicon and boron–tin double nucleophiles, including the arylation of the C–B bond with Ar–Cl, along with a complementary oxidative λ3-iodane-guided arylation of the C–Si/Sn moiety.
Chem. Sci., 2021,12, 10514-10521
https://doi.org/10.1039/D1SC01741A
Adapting decarbonylation chemistry for the development of prodrugs capable of in vivo delivery of carbon monoxide utilizing sweeteners as carrier molecules
1,2-Dicarbonyl compounds with FDA-approved sweeteners as leaving groups deliver CO for protection against acute kidney injury in mice.

Chem. Sci., 2021,12, 10649-10654
https://doi.org/10.1039/D1SC02711E
Photoinduced 1,2-dicarbofunctionalization of alkenes with organotrifluoroborate nucleophiles via radical/polar crossover
Alkene 1,2-dicarbofunctionalizations increase molecular complexity. Herein, the carboallylation, carboalkenylation, carboalkynylation, and carboarylation of olefins is accomplished through radical/polar crossover with organotrifluoroborates.

Chem. Sci., 2021,12, 9189-9195
https://doi.org/10.1039/D1SC02547C
Stable and easily available sulfide surrogates allow a stereoselective activation of alcohols
A simple and scalable method for stereoselective synthesis of thioethers directly from alcohols using isothiouronium salts is presented. The utility of this thiol-free reaction was exemplified by late-stage modification of complex molecules.
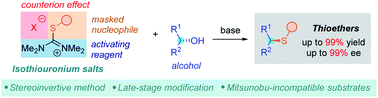
Chem. Sci., 2021,12, 7770-7774
https://doi.org/10.1039/D1SC01602D
Nickel-catalyzed asymmetric reductive cross-coupling of α-chloroesters with (hetero)aryl iodides
A Ni-catalyzed enantioselective reductive cross-coupling of α-chloroesters and (hetero)aryl iodides is reported. A MLR model was developed to quantitatively relate the influence of the α-chloroester substrate and ligand on enantioselectivity.
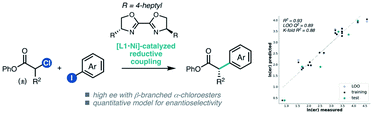
Chem. Sci., 2021,12, 7758-7762
https://doi.org/10.1039/D1SC00822F
Nickel-catalyzed asymmetric reductive aryl-allylation of unactivated alkenes
A nickel-catalyzed reductive asymmetric aryl-allylation of tethered unactivated alkenes has been developed, providing diverse benzene-annulated cyclic compounds bearing a quaternary stereocenter with high regio-, E/Z- and enantio-selectivity.

Chem. Sci., 2021,12, 6712-6718
https://doi.org/10.1039/D1SC01115D
Divergent reactivity of sulfinates with pyridinium salts based on one- versus two-electron pathways
Divergent reactions of sulfinates with pyridinium salts were developed by controlling the one- versus two-electron reaction manifolds.
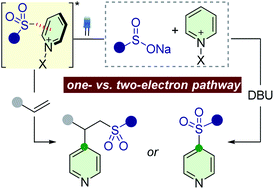
Chem. Sci., 2021,12, 6629-6637
https://doi.org/10.1039/D1SC00776A
Photocatalytic decarboxylative amidosulfonation enables direct transformation of carboxylic acids to sulfonamides
Sulfonamides are now accessible directly from carboxylic acids by a one-step, tricomponent decarboxylative amidosulfonation that provides the missing link between the two key functionalities.
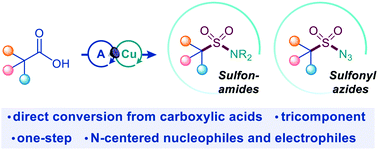
Chem. Sci., 2021,12, 6429-6436
https://doi.org/10.1039/D1SC01389K
Heterocyclic group transfer reactions with I(III) N-HVI reagents: access to N-alkyl(heteroaryl)onium salts via olefin aminolactonization
Complex N-alkyl (heteroaryl)onium salts are accessed via heterocyclic group transfer reactions of N-ligated I(iii) reagents with alkenoic acids. The reactions proceed in excellent yields, under mild conditions, and with broad substrate scope.
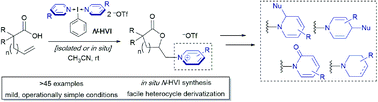
Chem. Sci., 2021,12, 6385-6392
https://doi.org/10.1039/D1SC00187F
Targeted 1,3-dipolar cycloaddition with acrolein for cancer prodrug activation
Prodrug activation strategy by utilizing the reaction between aryl azide and endogenous acrolein that is generally overproduced by cancer cells.
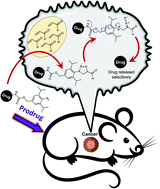
Chem. Sci., 2021,12, 5438-5449
https://doi.org/10.1039/D0SC06083F
Photoactive electron donor–acceptor complex platform for Ni-mediated C(sp3)–C(sp2) bond formation
This works demonstrates the implementation of an electron donor–acceptor (EDA) complex platform toward Ni-catalyzed C(sp3)–C(sp2) bond formation, circumventing the need for exogenous photocatalysts, additives, and stoichiometric metal reductants.
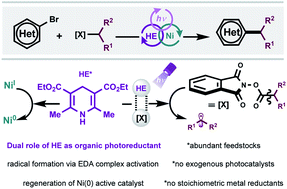
Chem. Sci., 2021,12, 5450-5457
https://doi.org/10.1039/D1SC00943E
Achieving high circularly polarized luminescence with push–pull helicenic systems: from rationalized design to top-emission CP-OLED applications
A CPL intensity of up to 3 × 10−2 is achieved in π-extended 6-helicene derivatives, owing to an intense helicene-mediated exciton coupling. Corresponding top-emission CP-OLEDs afforded a promising gEl of around 8 × 10−3.
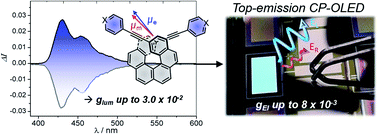
Chem. Sci., 2021,12, 5522-5533
https://doi.org/10.1039/D0SC06895K
Mutually exclusive hole and electron transfer coupling in cross stacked acenes
Acenes in the Greek cross (+) stack orientation exhibit selective hole and electron transfer coupling based on gerade symmetry in frontier molecular orbitals.
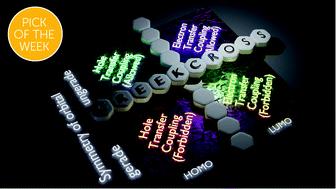
Chem. Sci., 2021,12, 5064-5072
https://doi.org/10.1039/D1SC00520K
Synthesis of quaternary centres by single electron reduction and alkylation of alkylsulfones
A new method for the generation of tertiary radicals through single electron reduction of alkylsulfones promoted by Zn and 1,10-phenanthroline has been developed.
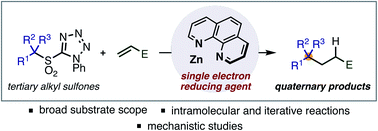
Chem. Sci., 2021,12, 4866-4871
https://doi.org/10.1039/D1SC00133G
Lewis acid mediated, mild C–H aminoalkylation of azoles via three component coupling
Lewis acid mediated activation enables mild, metal-free, and highly functional group tolerant C–H aminoalkylation of diverse azoles via three-component coupling.
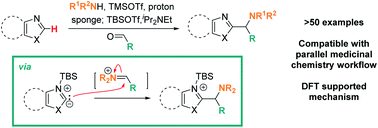
Chem. Sci., 2021,12, 3890-3897
https://doi.org/10.1039/D0SC06868C
Inhibitors of thiol-mediated uptake
Thiol-reactive inhibitors for the cellular entry of cyclic oligochalcogenide (COC) transporters and SARS-CoV-2 spike pseudo-lentivirus are reported.

Chem. Sci., 2021,12, 626-631
https://doi.org/10.1039/D0SC05447J
About this collection
This specially curated collection pulls together some of the most popular articles from 2021 in the field of organic chemistry. The collection presents some outstanding contributions to the field, ranging from C-H olefination to photosensitizers for cancer phototherapy, and as with all Chemical Science articles – they are all completely free to access and read. We hope you enjoy browsing through this collection.
If a particular article has inspired you, do feel free to share on social media using the buttons on each article landing page and use our hashtag: #ChemSciMostPopular