Nickel-catalyzed asymmetric reductive cross-coupling of α-chloroesters with (hetero)aryl iodides†
Abstract
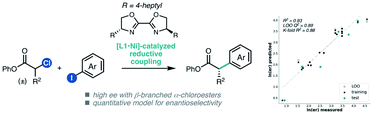
An asymmetric reductive cross-coupling of α-chloroesters and (hetero)aryl iodides is reported. This nickel-catalyzed reaction proceeds with a chiral BiOX ligand under mild conditions, affording α-arylesters in good yields and enantioselectivities. The reaction is tolerant of a variety of functional groups, and the resulting products can be converted to pharmaceutically-relevant chiral building blocks. A multivariate linear regression model was developed to quantitatively relate the influence of the α-chloroester substrate and ligand on enantioselectivity.
- This article is part of the themed collection: Most popular 2021 organic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...