Stable and easily available sulfide surrogates allow a stereoselective activation of alcohols†
Abstract
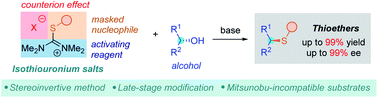
Isothiouronium salts are easily accessible and stable compounds. Herein, we report their use as versatile deoxasulfenylating agents enabling a stereoselective, thiol-free protocol for synthesis of thioethers from alcohols. The method is simple, scalable and tolerates a broad range of functional groups otherwise incompatible with other methods. Late-stage modification of several pharmaceuticals provides access to multiple analogues of biologically relevant molecules. Performed experiments give insight into the reaction mechanism.
- This article is part of the themed collection: Most popular 2021 organic chemistry articles, 2021


 Please wait while we load your content...
Please wait while we load your content...