Themed collection Glycosylation: New methodologies and applications

Introduction to Glycosylation: new methodologies and applications
M. Carmen Galan, Sabine Flitsch and Antony Fairbanks introduce the Organic & Biomolecular Chemistry themed collection on glycosylation: new methodologies and applications.

Org. Biomol. Chem., 2020,18, 6979-6982
https://doi.org/10.1039/D0OB90110E
Transition metal catalyzed glycosylation reactions – an overview
An overview of transition metal catalyzed glycosylation reactions is presented and the main trends in reactivity are discussed.
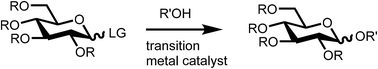
Org. Biomol. Chem., 2020,18, 9160-9180
https://doi.org/10.1039/D0OB01782E
Enzymatic glycosylation involving fluorinated carbohydrates
This contribution reviews the enzymatic synthesis, including optimisation efforts, of fluorinated carbohydrates involving fluorinated donors and/or acceptors, as well as the enzymatic activation of the fluorinated donors.

Org. Biomol. Chem., 2020,18, 3423-3451
https://doi.org/10.1039/D0OB00436G
Recent advances in β-L-rhamnosylation
This review describes various methodologies for the stereoselective 1,2-cis glycosylation of L-rhamnose leading to β-L-rhamnosides and their applications in oligosaccharide synthesis.
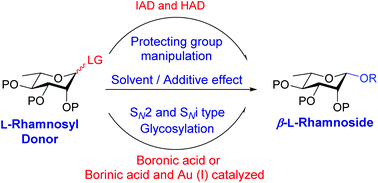
Org. Biomol. Chem., 2020,18, 3216-3228
https://doi.org/10.1039/D0OB00297F
Exploration of human xylosyltransferase for chemoenzymatic synthesis of proteoglycan linkage region
Human xylosyl transferase can effectively install a xylose stereospecifically onto a peptide backbone on mg scales paving the way for efficient chemoenzymatic synthesis of proteoglycan glycopeptides and glycoproteins.

Org. Biomol. Chem., 2021,19, 3374-3378
https://doi.org/10.1039/D1OB00317H
A protecting group–free approach for synthesizing C-glycosides through glycosyl dithiocarbamates
The first protection/deprotection-free process for radical C-glycosylation has been achieved through one-step preparable glycosyl dithiocarbamates (GDTCs).
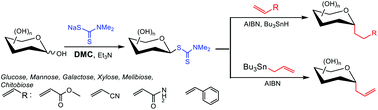
Org. Biomol. Chem., 2021,19, 3134-3138
https://doi.org/10.1039/D1OB00311A
Synthesis and evaluation of sensitive coumarin-based fluorogenic substrates for discovery of α-N-acetyl galactosaminidases through droplet-based screening
Synthesis of sensitive coumarin α-GalNAc glycosides as substrates for droplet-based screening of GalNAcases.
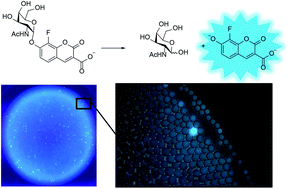
Org. Biomol. Chem., 2021,19, 789-793
https://doi.org/10.1039/D0OB02484H
Chemoselective activation of ethyl vs. phenyl thioglycosides: one-pot synthesis of oligosaccharides
Ethyl thioglycosides could be selectively activated in the presence of phenyl thioglycosides carrying the same or even more armed protecting group pattern.
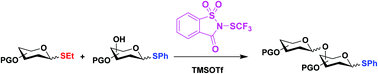
Org. Biomol. Chem., 2020,18, 9029-9034
https://doi.org/10.1039/D0OB01606C
Thioglycoligation of aromatic thiols using a natural glucuronide donor
This is the first example of a thioglycoligase that is able to catalyse the formation of S-glucuronides using aromatic thiols and a natural glucuronide donor.
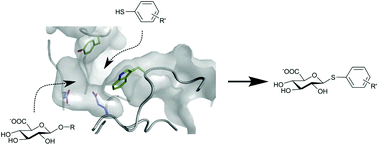
Org. Biomol. Chem., 2020,18, 5582-5585
https://doi.org/10.1039/D0OB00226G
Comparison of disaccharide donors for heparan sulfate synthesis: uronic acids vs. their pyranose equivalents
Disaccharide glycosyl donors were synthesised and directly compared in glycosylations to assess their potential for heparan sulfate oligosaccharide synthesis.
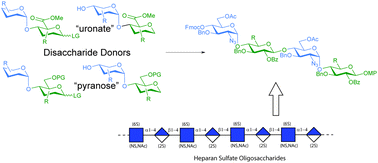
Org. Biomol. Chem., 2020,18, 4728-4733
https://doi.org/10.1039/D0OB00671H
Total synthesis of tricolorin A via interrupted Pummerer reaction-mediated glycosylation and one-pot relay glycosylation
The total synthesis of tricolorin A was achieved with high efficiency based on interrupted Pummerer reaction-mediated (IPRm) glycosylation and one-pot relay glycosylation.
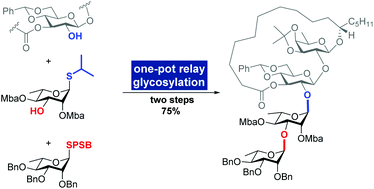
Org. Biomol. Chem., 2020,18, 3818-3822
https://doi.org/10.1039/D0OB00513D
Modular continuous flow synthesis of orthogonally protected 6-deoxy glucose glycals
The use of a continuous flow platform for the rapid and highly efficient construction of differentially protected glycals from commercial sources is described.
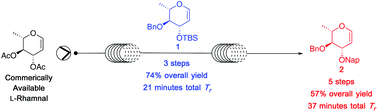
Org. Biomol. Chem., 2020,18, 3254-3257
https://doi.org/10.1039/D0OB00522C
Pseudo-enantiomeric carbohydrate-based N-heterocyclic carbenes as promising chiral ligands for enantiotopic discrimination
The practical synthesis of pseudo-enantiomeric carbohydrate-based NHC–Rh complexes bearing C1 or C3 sterically differentiated positions is reported. We show that steric bulk at either C1 or C3 leads to enantiotopic discrimination in the hydrosilylation of acetophenone.
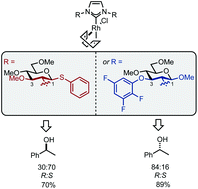
Org. Biomol. Chem., 2020,18, 3012-3016
https://doi.org/10.1039/D0OB00155D
C-Glycosylation enabled by N-(glycosyloxy)acetamides
An effective C-glycosylation protocol using N-(glycosyloxy)acetamides as donors promoted by SnBr4 has been developed, delivering alkyl C-glycosides and aryl-β-C-glycosides in high yields.
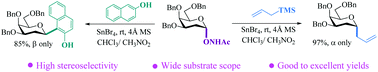
Org. Biomol. Chem., 2020,18, 3043-3046
https://doi.org/10.1039/D0OB00561D
Synthesis of orthogonally protected and functionalized bacillosamines
The synthesis of bacillosamines carrying different functionalities at their C-2 and C-4 amine groups is described for the first time.
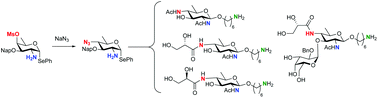
Org. Biomol. Chem., 2020,18, 2834-2837
https://doi.org/10.1039/D0OB00256A
1,2-cis-Selective glucosylation enabled by halogenated benzyl protecting groups
Electron-withdrawing groups and Lewis basic solvent enable 1,2-cis-selectivity.
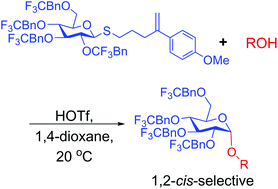
Org. Biomol. Chem., 2020,18, 2405-2409
https://doi.org/10.1039/D0OB00373E
Reagent controlled stereoselective synthesis of teichoic acid α-(1,2)-glucans
Additive controlled glycosylation reactions are used for the construction of α-(1,2)-glucosidic linkages, such as those featuring in E. faecalis lipoteichoic acid.
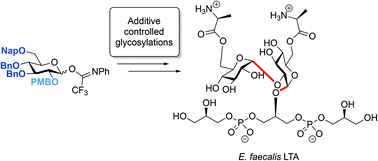
Org. Biomol. Chem., 2020,18, 2038-2050
https://doi.org/10.1039/D0OB00240B
Automated access to well-defined ionic oligosaccharides
Automated Glycan Assembly grants access to ionic oligosaccharides for structural studies.

Org. Biomol. Chem., 2020,18, 1349-1353
https://doi.org/10.1039/D0OB00137F
Photo-induced glycosylation using a diaryldisulfide as an organo-Lewis photoacid catalyst
Photo-induced glycosylations of several acceptors with trichloroacetimidate donors using bis(2-naphthyl)disulfide as an organo-Lewis photoacid (LPA) catalyst proceeded effectively to give the corresponding glycosides in good to high yields.
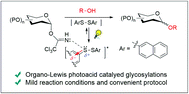
Org. Biomol. Chem., 2020,18, 851-855
https://doi.org/10.1039/C9OB02674F
A hybrid polymer to target blood group dependence of cholera toxin
New hybrid glycopolymers were synthesized that contain two epitopes blocking GM1- and fucose-based intoxication modes of the cholera toxin.
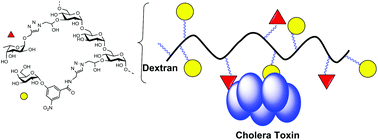
Org. Biomol. Chem., 2020,18, 52-55
https://doi.org/10.1039/C9OB02369K
Synthesis and immunological evaluation of the unnatural β-linked mucin-1 Thomsen–Friedenreich conjugate
A MUC1 glycopeptide bearing an unnatural β-glycosyl bond between the glycan and the peptide backbone was synthesized. The mimic can induce high levels of IgG antibodies cross-recognizing cancer cells expressing the native MUC1 glycoprotein.
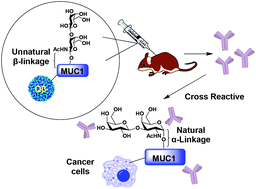
Org. Biomol. Chem., 2021,19, 2448-2455
https://doi.org/10.1039/D1OB00007A
Scope of the DMC mediated glycosylation of unprotected sugars with phenols in aqueous solution
Activation of reducing sugars in aqueous solution using DMC and triethylamine in the presence of phenols allows direct stereoselective conversion to the corresponding 1,2-trans aryl glycosides without the need for any protecting groups.
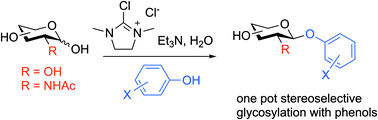
Org. Biomol. Chem., 2020,18, 7355-7365
https://doi.org/10.1039/D0OB01727B
Synthesis of an STnThr analogue, structurally based on a TnThr antigen mimetic
The monosaccharide Tn and the disaccharide STn are tumor antigens with similar structures and common biosynthetic pathways.
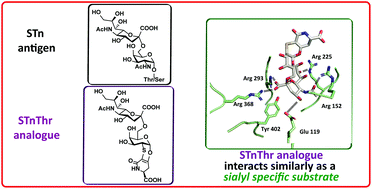
Org. Biomol. Chem., 2020,18, 7366-7372
https://doi.org/10.1039/D0OB01749C
Synthesis of hyaluronic acid oligosaccharides with a GlcNAc–GlcA repeating pattern and their binding affinity with CD44
A practical route for the synthesis of hyaluronic acid oligosaccharides was developed, and a tetrasaccharide (GlcNAc–GlcA)2 was identified as the minimum length that binds to human CD44 (KD = 3.5 μM) using isothermal titration calorimetry.
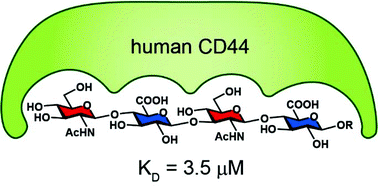
Org. Biomol. Chem., 2020,18, 5370-5387
https://doi.org/10.1039/D0OB01048K
Solvent-free, under air selective synthesis of α-glycosides adopting glycosyl chlorides as donors
A solvent-free, under air approach for the highly stereoselective synthesis of α-glycosides from glycosyl chloride donors, promoted by a triethylphosphite, tetrabutylammonium bromide, and N,N-diisopropylethylamine combination.
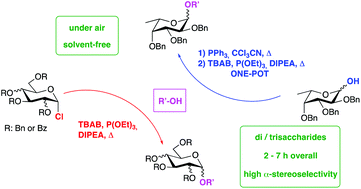
Org. Biomol. Chem., 2020,18, 5157-5163
https://doi.org/10.1039/D0OB01024C
Synthesis of oligosaccharides of the linkage region of proteoglycans using regioselective glycosylation
A collection of various sulfoforms of di- and trisaccharides of the linkage region of proteoglycans were prepared using a regioselective glycosylation. Preliminary results of the impact of sulfation on CSGalNAcT-1 is also reported.
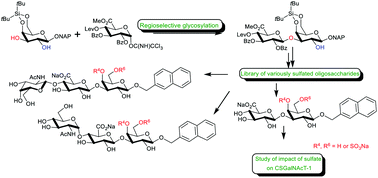
Org. Biomol. Chem., 2020,18, 4831-4842
https://doi.org/10.1039/D0OB00783H
Synthesis of type 1 Lewis b hexasaccharide antigen structures featuring flexible incorporation of L-[U-13C6]-fucose for NMR binding studies
The synthesis and use of a uniformly 13C labelled fucosyl donor as a general building block is demonstrated.
![Graphical abstract: Synthesis of type 1 Lewis b hexasaccharide antigen structures featuring flexible incorporation of l-[U-13C6]-fucose for NMR binding studies](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0OB00426J&imageInfo.ImageIdentifier.Year=2020)
Org. Biomol. Chem., 2020,18, 4452-4458
https://doi.org/10.1039/D0OB00426J
Asymmetric trehalose analogues to probe disaccharide processing pathways in mycobacteria
Chemoenzymatic synthesis of azido-functionalised asymmetric trehalose analogues that are resistant to enzymatic degradation to probe carbohydrate processing pathways in mycobacteria.
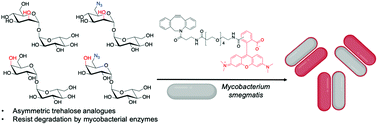
Org. Biomol. Chem., 2020,18, 3607-3612
https://doi.org/10.1039/D0OB00253D
Sub-stoichiometric reductive etherification of carbohydrate substrates and one-pot protecting group manipulation
Polymethylhydrosiloxane (PMHS): a sub-stoichiometric reducing agent for reductive etherification of carbohydrate substrates and its application for one-pot protecting group manipulation
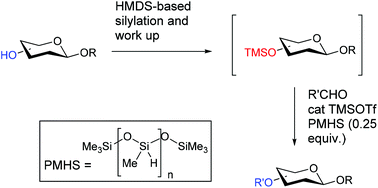
Org. Biomol. Chem., 2020,18, 3135-3141
https://doi.org/10.1039/D0OB00252F
Biochemical characterisation of an α1,4 galactosyltransferase from Neisseria weaveri for the synthesis of α1,4-linked galactosides
A new α1,4 galactosyltransferase has been characterised and used for the synthesis of natural and non-natural cell surface trisaccharide antigens.
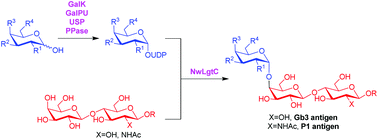
Org. Biomol. Chem., 2020,18, 3142-3148
https://doi.org/10.1039/D0OB00407C
Synthesis and evaluation of Nα,Nε-diacetyl-L-lysine-inositol conjugates as cancer-selective probes for metabolic engineering of GPIs and GPI-anchored proteins
This study has established a new strategy and new molecular tools for selective metabolic labeling of inositol on cancer cells, which should be useful for cancer targeting and study of GPI-anchored proteins.
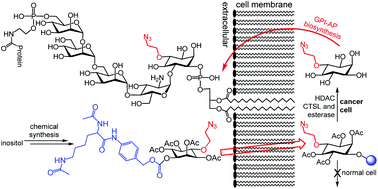
Org. Biomol. Chem., 2020,18, 2938-2948
https://doi.org/10.1039/D0OB00333F
Efficient diversification of GM3 gangliosides via late-stage sialylation and dynamic glycan structural studies with 19F solid-state NMR
GM3 gangliosides have been synthesized via late-stage α-sialylation using a macro-bicyclic sialyl donor. 19F solid-state NMR analysis of the C5-NHTFAc GM3 analog on a model membrane revealed the influence of cholesterol on glycan dynamics.
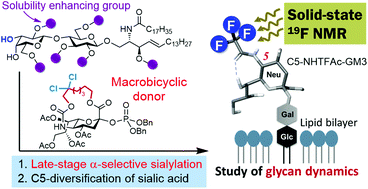
Org. Biomol. Chem., 2020,18, 2902-2913
https://doi.org/10.1039/D0OB00437E
Synthetic preparation and immunological evaluation of β-mannosylceramide and related N-acyl analogues
The synthesis of the invariant natural killer (iNK) T cell agonist β-mannosylceramide along with a series of fatty amide analogues is reported.

Org. Biomol. Chem., 2020,18, 2739-2746
https://doi.org/10.1039/D0OB00223B
S-Glycosides: synthesis of S-linked arabinoxylan oligosaccharides
An S-linked disaccharide for the efficient synthesis of arabinoxylans.
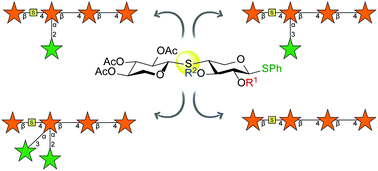
Org. Biomol. Chem., 2020,18, 2696-2701
https://doi.org/10.1039/D0OB00470G
Palladium(II)-catalyzed stereoselective synthesis of C-glycosides from glycals with diaryliodonium salts
An effective Pd(II)-catalyzed stereoselective C-glycosylation method has been successfully manifested for the synthesis of diversely functionalized 2,3-dideoxy C-aryl glycosides starting from glycals with a wide range of diaryliodonium salts.
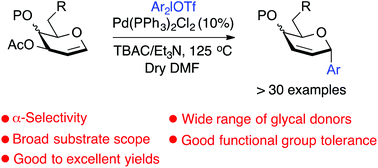
Org. Biomol. Chem., 2020,18, 2242-2251
https://doi.org/10.1039/D0OB00247J
β-Selective xylulofuranosylation via a conformationally-restricted glycosyl donor
Reported is the first stereoselective method for β-xylulofuranosylation, which employs 3,4-O-xylylene-protected thioglycoside donors.

Org. Biomol. Chem., 2020,18, 2264-2273
https://doi.org/10.1039/D0OB00260G
α-Selective glycosylations using glycosyl N-(ortho-methoxyphenyl)trifluoroacetimidates
β-Selective acetimidate formation followed by α-selective catalytic activation.
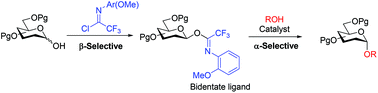
Org. Biomol. Chem., 2020,18, 1918-1925
https://doi.org/10.1039/C9OB02696G
Chemical synthesis of human milk oligosaccharides: lacto-N-neohexaose (Galβ1 → 4GlcNAcβ1→)2 3,6Galβ1 → 4Glc
The first chemical synthesis of lacto-N-neohexaose (LNnH) has been completed using a convergent synthetic strategy.
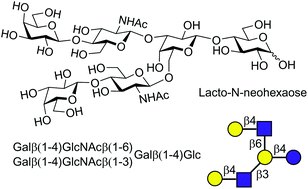
Org. Biomol. Chem., 2020,18, 1747-1753
https://doi.org/10.1039/D0OB00172D
C-2 auxiliaries for stereoselective glycosylation based on common additive functional groups
The stereoselective introduction of the glycosidic bond is one of the main challenges in chemical oligosaccharide synthesis.
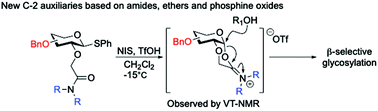
Org. Biomol. Chem., 2020,18, 1165-1184
https://doi.org/10.1039/C9OB02700A
L. pneumophila CMP-5,7-di-N-acetyllegionaminic acid synthetase (LpCLS)-involved chemoenzymatic synthesis of sialosides and analogues
A bacterial CMP-5,7-di-N-acetyllegionaminic acid synthetase was characterized and used in one-pot multienzyme systems for efficient synthesis of Leg5,7Ac2-glycosides and analogs.
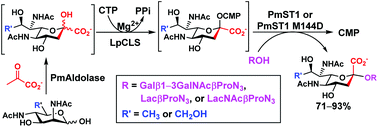
Org. Biomol. Chem., 2020,18, 738-744
https://doi.org/10.1039/C9OB02476J
Exploring a glycosylation methodology for the synthesis of hydroxamate-modified alginate building blocks
Mixed sequence, C6-hydroxamate-modified alginate disaccharides are prepared using NIS/TMSOTf glycosylation.
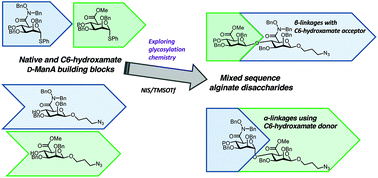
Org. Biomol. Chem., 2019,17, 9321-9335
https://doi.org/10.1039/C9OB02053E
Melioidosis patient serum-reactive synthetic tetrasaccharides bearing the predominant epitopes of Burkholderia pseudomallei and Burkholderia mallei O-antigens
Tetrasaccharides mimicking Burkholderia pseudomallei and Burkholderia mallei lipopolysaccharide O-antigens were synthesized and found to be highly reactive with Thai melioidosis patient serum, highlighting their potential as vaccine candidates.
Org. Biomol. Chem., 2019,17, 8878-8901
https://doi.org/10.1039/C9OB01711A
About this collection
Glycosylation: New methodologies for oligosaccharide and glycoconjugate synthesis and their applications
This themed collection, Guest Edited by Professors M. Carmen Galan, Sabine Flitsch and Antony Fairbanks, is to highlight the exciting work being carried out by carbohydrate researchers at the forefront of their field. The scope of this collection includes reactions of the anomeric centre, di- and oligosaccharide synthesis, and the development of synthetic methodology for the production of glycoconjugates for biological, biophysical or other study.
Articles in this themed collection will be added below as soon as possible after they are published.
Please return to this page frequently to see the collection grow.