α-Selective glycosylations using glycosyl N-(ortho-methoxyphenyl)trifluoroacetimidates†
Abstract
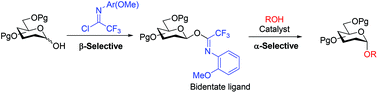
Six N-(o-methoxyphenyl)trifluoroacetimidate glycosyl donors have been synthesized and their role as glycosyl donors has been investigated. The donors were synthesized with complete β-selectivity, except in one case, and were found to be stable. When Bi(OTf)3, Fe(OTf)2, and Zn(OTf)2 were employed as catalysts, the glycosylations were found to be highly α-selective in Et2O. The selectivity and reaction rate changed with a change in the acceptor reactivity.
- This article is part of the themed collection: Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...