Solvent-free, under air selective synthesis of α-glycosides adopting glycosyl chlorides as donors†
Abstract
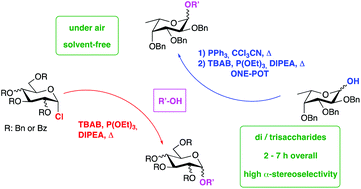
α-Glycosides are highly relevant synthetic targets due to their abundance in natural oligosaccharides involved in many biological processes. Nevertheless their preparation is hampered by several issues, due to both the strictly anhydrous conditions typically required in glycosylation procedures and the non-trivial achievement of high α-stereoselectivity, one of the major challenges in oligosaccharide synthesis. In this paper we report a novel and efficient approach for the highly stereoselective synthesis of α-glycosides. This is based on the unprecedented solvent-free combination of triethylphosphite, tetrabutylammonium bromide and N,N-diisopropylethylamine for the activation of glycosyl chlorides under air. Despite the relative stability of glycosyl chlorides with respect to more reactive halide donors, the solvent-free procedure allowed a wide set of α-glycosides, including biorelevant fragments, to be obtained in much shorter times compared with similar glycosylation approaches in solution. The presented method features a wide target scope and functional group compatibility, also serving with partially disarmed substrates, and it does not require a high stoichiometric excess of reagents nor the preparation of expensive precursors. The solvent-free glycosylation can be even directly performed from 1-hydroxy sugars without purification of the in situ generated chloride, providing an especially useful opportunity in the case of highly reactive and labile glycosyl donors.
- This article is part of the themed collections: Chemical Biology in OBC and Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...