A protecting group–free approach for synthesizing C-glycosides through glycosyl dithiocarbamates†
Abstract
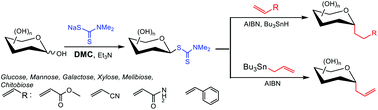
The first protection/deprotection-free process for radical C-glycosylation has been achieved through one-step preparable glycosyl dithiocarbamates (GDTCs). The Giese-type reaction and radical allylation of unprotected GDTCs were successfully performed to obtain the corresponding α-C-glycosides stereoselectively under mild reaction conditions.
- This article is part of the themed collections: Chemical Biology in OBC and Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...