Scope of the DMC mediated glycosylation of unprotected sugars with phenols in aqueous solution†
Abstract
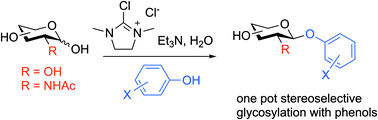
Activation of reducing sugars in aqueous solution using 2-chloro-1,3-dimethylimidazolinium chloride (DMC) and triethylamine in the presence of para-nitrophenol allows direct stereoselective conversion to the corresponding 1,2-trans para-nitrophenyl glycosides without the need for any protecting groups. The reaction is applicable to sulfated and phosphorylated sugars, but not to ketoses or uronic acids or their derivatives. When applied to other phenols the product yield was found to depend on the pKa of the added phenol, and the process was less widely applicable to 2-acetamido sugars. For 2-acetamido substrates an alternative procedure in which the glycosyl oxazoline was pre-formed, the reaction mixture freeze-dried, and the crude product then reacted with an added phenol in a polar aprotic solvent system with microwave irradiation proved to be a useful simplification.
- This article is part of the themed collection: Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...