Exploring a glycosylation methodology for the synthesis of hydroxamate-modified alginate building blocks†
Abstract
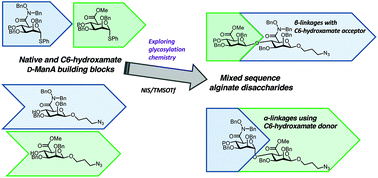
Alginate, an anionic polysaccharide, is an important industrial biomaterial naturally harvested from seaweed. Many of its important physicochemical properties derive from the presence of charged carboxylate groups presented as uronic acids within the polysaccharide backbone. An ability to modify these carboxylates with alternate functional groups would enable the design and implementation of new alginate systems possessing different physicochemical properties. We present herein our approach to the chemical synthesis of alginate disaccharides, modified at the carboxylate C6 position with bioisosteric hydroxamate residues. The synthesis and utilisation of C6-hydroxamate donor and acceptor building blocks enables a first access to protected α- and β-linked hydroxamate-containing disaccharides, additionally equipped with a functional tether at the reducing terminus. The evaluation of these building blocks for chemical glycosylation demonstrates the incorporation of bioisosteric functional groups into an alginate disaccharide backbone and highlights the important contribution of both acceptor and donor reactivity underpinning uronate glycosylations.
- This article is part of the themed collections: Chemical Biology in OBC and Glycosylation: New methodologies and applications


 Please wait while we load your content...
Please wait while we load your content...