Themed collection Amyloid Aggregation

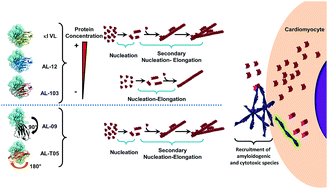
Immunoglobulin light chain amyloid aggregation
Light chain (AL) amyloidosis is a devastating, complex, and incurable protein misfolding disease.
Chem. Commun., 2018,54, 10664-10674
https://doi.org/10.1039/C8CC04396E
Secondary nucleation in amyloid formation
Nucleation of new peptide and protein aggregates on the surfaces of amyloid fibrils of the same peptide or protein has emerged in the past two decades as a major pathway for both the generation of molecular species responsible for cellular toxicity and for the autocatalytic proliferation of peptide and protein aggregates.

Chem. Commun., 2018,54, 8667-8684
https://doi.org/10.1039/C8CC02204F
Structure, function and antagonism of semen amyloids
Amyloids in semen enhance HIV infection and promote clearance of defect sperm.
Chem. Commun., 2018,54, 7557-7569
https://doi.org/10.1039/C8CC01491D
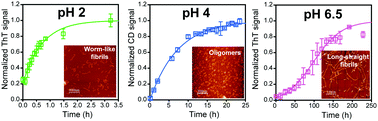
Structural mechanisms of oligomer and amyloid fibril formation by the prion protein
The aggregation mechanism of the prion protein is highly heterogeneous.
Chem. Commun., 2018,54, 6230-6242
https://doi.org/10.1039/C8CC03053G
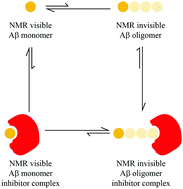
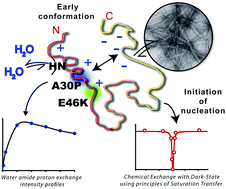
A solution NMR toolset to probe the molecular mechanisms of amyloid inhibitors
A chemical exchange-based solution NMR toolset to probe the molecular mechanisms of amyloid inhibitors.
Chem. Commun., 2018,54, 4644-4652
https://doi.org/10.1039/C8CC01380B
Synthesis and biochemical characterization of quasi-stable trimer models of full-length amyloid β40 with a toxic conformation
The only trimer model to exhibit weak but significant neurotoxicity against SH-SY5Y cells was the one which was linked at position 38.
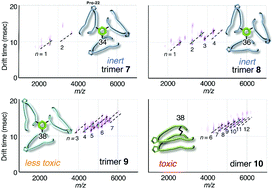
Chem. Commun., 2019,55, 182-185
https://doi.org/10.1039/C8CC08618D
Structural differences between toxic and nontoxic HypF-N oligomers
We have studied two misfolded oligomeric forms of the protein HypF-N, which show similar morphologies but very different toxicities.
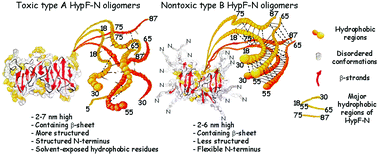
Chem. Commun., 2018,54, 8637-8640
https://doi.org/10.1039/C8CC03446J
Probing the early stages of prion protein (PrP) aggregation with atomistic molecular dynamics simulations
We present results extracted from molecular dynamics simulations aimed at investigating the aggregation process of the β-rich ovine prion protein.
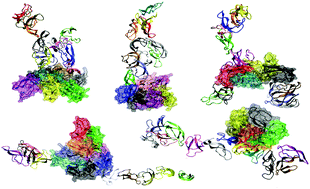
Chem. Commun., 2018,54, 8007-8010
https://doi.org/10.1039/C8CC04089C
Energy landscape of polymorphic amyloid generation of β2-microglobulin revealed by calorimetry
ITC-based energy landscape provides a comprehensive understanding of amyloid aggregation in terms of thermodynamics and kinetics.
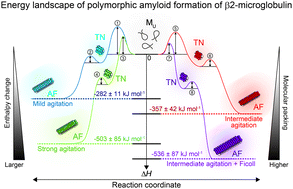
Chem. Commun., 2018,54, 7995-7998
https://doi.org/10.1039/C8CC02718H
Chiral modulation of amyloid beta fibrillation and cytotoxicity by enantiomeric carbon dots
Enantiomeric carbon dots (C-dots) synthesized from L-lysine or D-lysine, modulate aggregation and cytotoxicity of amyloid beta-42 (Aβ42), the primary constituent of the amyloid plaques associated with Alzheimer's disease.
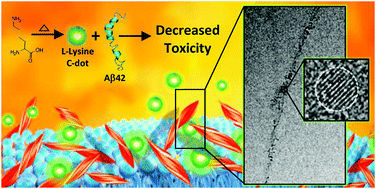
Chem. Commun., 2018,54, 7762-7765
https://doi.org/10.1039/C8CC03235A
Aβ under stress: the effects of acidosis, Cu2+-binding, and oxidation on amyloid β-peptide dimers
In light of the high affinity of Cu2+ for Alzheimer's Aβ1–42 and its ability to subsequently catalyze the formation of radicals, we examine the effects of Cu2+ binding, Aβ oxidation, and an acidic environment on the conformational dynamics of the smallest Aβ1–42 oligomer, the Aβ1–42 dimer.
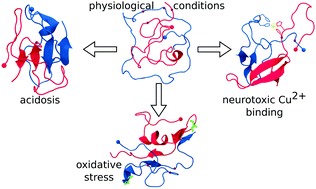
Chem. Commun., 2018,54, 7766-7769
https://doi.org/10.1039/C8CC02263A
Fluorescence quenching by lipid encased nanoparticles shows that amyloid-β has a preferred orientation in the membrane
Short range plasmonic fields around a nanoparticle can modulate fluorescence or Raman processes.
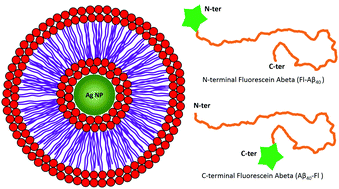
Chem. Commun., 2018,54, 7750-7753
https://doi.org/10.1039/C8CC02108B
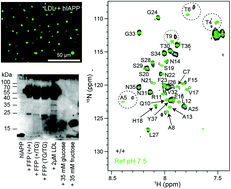
Aggregation kinetics of the Aβ1–40 peptide monitored by NMR
The aggregation of Aβ1–40 was monitored by solution NMR, which showed a trend complementary to the one observed by ThT-fluorescence.
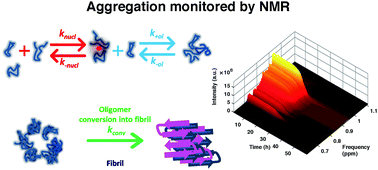
Chem. Commun., 2018,54, 7601-7604
https://doi.org/10.1039/C8CC01710G
Raman fingerprints of amyloid structures
Amyloids have well-ordered β-strands with aligned amide bonds and strong coupled vibrational modes, measurable by Raman microspectroscopy.
Chem. Commun., 2018,54, 6983-6986
https://doi.org/10.1039/C8CC03217C
Role of the carboxy groups of triterpenoids in their inhibition of the nucleation of amyloid β42 required for forming toxic oligomers
The carboxy group of ursane-type triterpenoids plays a critical role in the suppression of toxic Aβ42 nucleation by targeting the monomer to trimer.
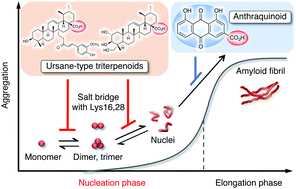
Chem. Commun., 2018,54, 6272-6275
https://doi.org/10.1039/C8CC03230K
Inhibition of tau-derived hexapeptide aggregation and toxicity by a self-assembled cyclic D,L-α-peptide conformational inhibitor
Self-assembled cyclic D,L-α-peptide CP-2 cross-interacts with tau-derived AcPHF6 peptide to inhibit its aggregation, membrane perturbation and toxicity.
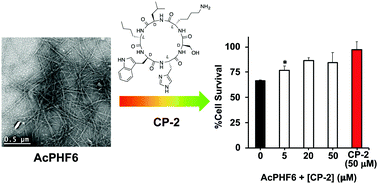
Chem. Commun., 2018,54, 5980-5983
https://doi.org/10.1039/C8CC01233D
The distinct structural preferences of tau protein repeat domains
Among the four bi-repeat protofilaments, only R3–R4 can maintain the C-shaped structure that can be stabilized by heparins, while R1–R2 tends to be linear in shape, and these two structural motifs appear in the most stable K18 protofilament.
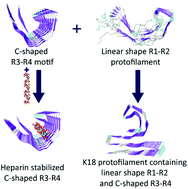
Chem. Commun., 2018,54, 5700-5703
https://doi.org/10.1039/C8CC01263F
Pressure and cosolvent modulation of the catalytic activity of amyloid fibrils
We report on the effects of pressure and cosolvents on the catalytic activity of a designed amyloid fibril by applying a high-pressure stopped-flow methodology with rapid spectroscopic detection.
Chem. Commun., 2018,54, 5696-5699
https://doi.org/10.1039/C8CC00699G
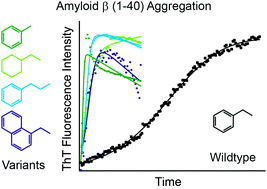
Ring structure modifications of phenylalanine 19 increase fibrillation kinetics and reduce toxicity of amyloid β (1–40)
Putatively minor alterations of the side chain ring of phenylalanine 19 of Aβ strongly influence fibril formation kinetics and toxicity.
Chem. Commun., 2018,54, 5430-5433
https://doi.org/10.1039/C8CC01733F
hIAPP forms toxic oligomers in plasma
Glucose and lipid containing particles such as LDL interact with hIAPP, resulting in the formation of hIAPP oligomeric structures that yield an intrinsic fluorescence and toxicity in cellular assays.
Chem. Commun., 2018,54, 5426-5429
https://doi.org/10.1039/C8CC03097A
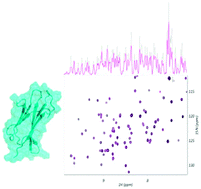
Interference of citrate-stabilized gold nanoparticles with β2-microglobulin oligomeric association
Citrate-coated gold nanoparticles interfere with the association equilibria of β2-microglobulin and thus inhibit the early events of fibrillogenesis.
Chem. Commun., 2018,54, 5422-5425
https://doi.org/10.1039/C8CC01053F
Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue
Only about half of the 40-residue amyloid-β sequence is structurally ordered in a common fibril structure from Alzheimer's disease brain.
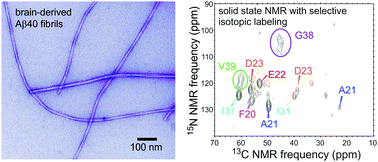
Chem. Commun., 2018,54, 5070-5073
https://doi.org/10.1039/C8CC01967C
Metabolite amyloid-like fibrils interact with model membranes
Metabolite assemblies interaction with membranes further extend the “amyloid hypothesis” to include small metabolites which serve as amyloidogenic building blocks.
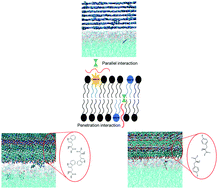
Chem. Commun., 2018,54, 4561-4564
https://doi.org/10.1039/C8CC01423J
High pressure NMR reveals conformational perturbations by disease-causing mutations in amyloid β-peptide
High pressure NMR reveals conformational biases in disease-causing variants of the Aβ monomer.
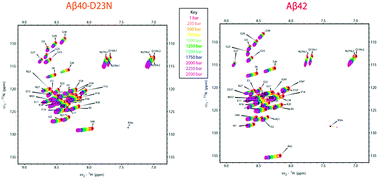
Chem. Commun., 2018,54, 4609-4612
https://doi.org/10.1039/C8CC01674G
Heparin-induced tau filaments are structurally heterogeneous and differ from Alzheimer's disease filaments
Tau filaments formed with heparin are structurally different from Alzheimer's disease filaments extracted from human brains. Heparin creates heterogeneous filaments in which tau proteins are locally stretched and have minimal large-domain structuration.
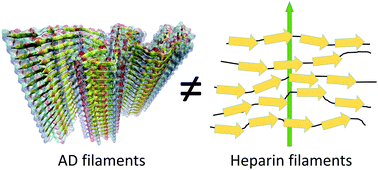
Chem. Commun., 2018,54, 4573-4576
https://doi.org/10.1039/C8CC01355A
Investigations of dynamic amyloid-like structures of the Wnt signalling pathway by solid-state NMR
Solid-state nuclear magnetic resonance can reveal native structural details of amyloid-like signalling proteins of the Wnt pathway.
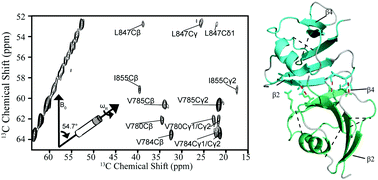
Chem. Commun., 2018,54, 3959-3962
https://doi.org/10.1039/C8CC01346B
Multitude NMR studies of α-synuclein familial mutants: probing their differential aggregation propensities
Familial mutations in α-synuclein affect the immediate chemical environment of the protein's backbone, changing its aggregation kinetics and forming diverse structural and functional intermediates.
Chem. Commun., 2018,54, 3605-3608
https://doi.org/10.1039/C7CC09597J
Serum amyloid A sequesters diverse phospholipids and their hydrolytic products, hampering fibril formation and proteolysis in a lipid-dependent manner
Serum amyloid A can solubilize diverse phospholipids and their hydrolytic products to form lipoprotein nanoparticles, which hampers amyloid fibril formation.
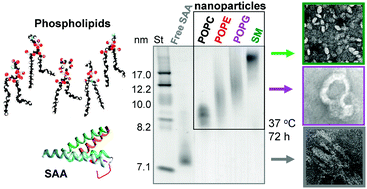
Chem. Commun., 2018,54, 3532-3535
https://doi.org/10.1039/C8CC01424H
Inhibition of amyloid Aβ aggregation by high pressures or specific D-enantiomeric peptides
Pressure as well as specific D-enantiomeric peptides can stabilize Aβ-monomers and thus inhibit amyloid aggregation.
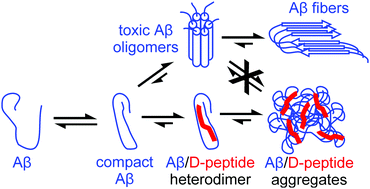
Chem. Commun., 2018,54, 3294-3297
https://doi.org/10.1039/C8CC01458B
About this collection
This web themed issue is guest edited by Professor Ehud Gazit (Tel Aviv University) and Professor Ayyalusamy Ramamoorthy (University of Michigan), and showcases recent amyloid-related advances from kinetic studies on amyloid assemblies to biophysical techniques to characterize structures. There have been recent advances in theoretical, computational and experimental studies to better characterize the amyloid aggregation process, and this collection aims to highlight further developments. Growth in this important field will bring scientists closer to treating amyloid diseases such as Type II diabetes, Alzheimer’s, Huntingdon’s and Parkinson’s.
Articles in this web themed issue will be added below as soon as possible after they are published. Please return to this page frequently to see the collection grow.