Coexisting order and disorder within a common 40-residue amyloid-β fibril structure in Alzheimer's disease brain tissue†
Abstract
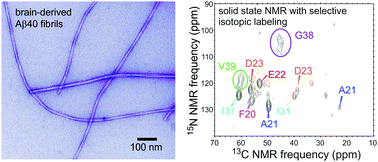
Fibrils formed by 40- and 42-residue amyloid-β (Aβ40 and Aβ42) peptides exhibit molecular-level structural polymorphisms. A recent screen of fibrils derived from brain tissue of Alzheimer's disease patients revealed a single predominant Aβ40 polymorph. We present solid state nuclear magnetic resonance (ssNMR) data that define its coexisting structurally ordered and disordered segments.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...