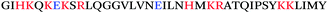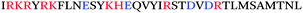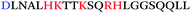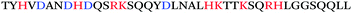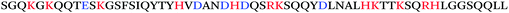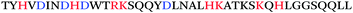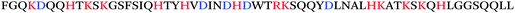Open Access Article
Open Access ArticleStructure, function and antagonism of semen amyloids
Annika
Röcker
a,
Nadia R.
Roan
bc,
Jay Kant
Yadav
de,
Marcus
Fändrich
d and
Jan
Münch
 *af
*af
aInstitute of Molecular Virology, Ulm University Medical Center, 89081 Ulm, Germany. E-mail: jan.muench@uni-ulm.de; Tel: +49 731 5500 65154
bDepartment of Urology, University of California, San Francisco, San Francisco, CA 94158, USA
cGladstone Institute of Virology and Immunology, San Francisco, CA 94158, USA
dInstitute of Protein Biochemistry, Ulm University, Helmholtzstr. 8/1, 89081 Ulm, Germany
eDepartment of Biotechnology, Central University of Rajasthan, NH-8 Bandarsindri, Kishangarh, Ajmer, India
fCore Facility Functional Peptidomics, Ulm University Medical Center, Ulm, Germany
First published on 6th June 2018
Abstract
Amyloid fibrils are linear polypeptide aggregates with a cross-β structure. These fibrils are best known for their association with neurodegenerative diseases, such as Alzheimer's or Parkinson's, but they may also be used by living organisms as functional units, e.g. in the synthesis of melanin or in the formation of bacterial biofilms. About a decade ago, in a search for semen factors that modulate infection by HIV-1 (a sexually transmitted virus and the causative agent of the acquired immune deficiency syndrome (AIDS)), it was demonstrated that semen harbors amyloid fibrils capable of markedly increasing HIV infection rates. This discovery not only created novel opportunities to prevent sexual HIV-1 transmission but also stimulated research to unravel the natural role of these factors. We discuss here the identification of these intriguing structures, their molecular properties, and their effects on both sexually transmitted diseases and reproductive health. Moreover, we review strategies to antagonize semen amyloid to prevent sexual transmission of viruses.
1. Seminal plasma: more than just a sperm vehicle
Seminal plasma (SP) is the liquid fraction of semen produced by the male accessory sex organs. SP, far from simply serving as a passive vehicle to transport sperm during conception, harbours many bioactive agents that promote reproductive success. In the absence of the protective effects of SP, oxidative damage to sperm can occur.1 SP also elicits responses in the female reproductive tract (FRT) that promote conception. Studies in mice have demonstrated that the signalling effects of SP can be long-lasting, spanning from the peri-conception period all the way to post-birth. In particular, the absence of SP during mating decreased the rate of conception, and mice that were conceived despite the absence of SP exhibited metabolic abnormalities relative to mice that were conceived in the presence of SP.2 In humans, SP can increase the rate of implantation, both when introduced through capsules and other artificial means and when introduced via sexual intercourse.3 At the molecular level, SP is a strong inducer of various signal transduction pathways, and elicits a potent and rapid transcriptional response characterized by a gene expression signature associated with inflammation, cellular migration, proliferation, and viability.4–6 Perhaps not surprisingly, SP also affects infection by sexually transmitted pathogenic microbes, through both direct (e.g., anti-bacterial peptides) and indirect (e.g., by eliciting a pro-inflammatory response in the FRT) means. The complex effects of SP on reproduction and infection by seminal microbes can be mediated through a plethora of different SP constituents, such as the thousands of different proteins, sugars, and lipids present in SP, in addition to the cargo of highly abundant exosomes of which trillions are present in a typical ejaculate.72. Identification of semen amyloids
The initial discovery that semen harbors amyloid-forming peptides that boost HIV infectivity was made rather serendipitously. In order to discover molecules in human semen modulating HIV infection, Münch et al. analysed a library of peptides and proteins derived from pooled human semen. In contrast to similar studies conducted using libraries generated from hemofiltrate,8,9 which had identified inhibitors of HIV infection, screening of the semen-derived fractions identified two fractions that potently increased HIV infection rates.10 Mass spectrometric analysis of the dominant fraction identified a series of peptides derived from the C-terminal region of prostatic acid phosphatase (PAP), with the dominant peptide being comprised of residues 248–286 (Table 1). PAP is a homodimeric enzyme that is secreted from the epithelial cells of the prostate gland, and is present at high concentrations (>1 mg mL−1) in semen.11 Freshly synthesized PAP(248–286) did not enhance HIV infection. Intriguingly, however, when PAP(248–286) was agitated, the peptide polymerized into amyloid fibrils (Fig. 1) that potently increased HIV infection rates – by up to several orders of magnitude. These fibrils were termed SEVI, for Semen-derived Enhancer of Viral Infection.10 SEVI was demonstrated to show key characteristics of amyloid fibrils through a variety of biochemical and biophysical measurements (see below).Additional studies subsequently revealed that SEVI was not the only amyloid species in human semen. Analysis of the second semen-derived fraction harbouring infection-enhancing activity revealed a second fragment of PAP, PAP(85–120), that, like SEVI, formed fibrils that enhanced HIV infection of cellular targets (Table 1).12
Using a completely different approach where antibodies recognizing the general amyloid fold were used to fish out endogenous amyloids from human semen, Roan et al. identified a second class of semen amyloids, this time derived from the two highly homologous semen proteins semenogelin-1 and semenogelin-2 (Table 1).13,14 This second class of amyloids, collectively termed “SEM amyloids”, exhibited no sequence homology to either PAP(248–286) or PAP(85–120), but exhibited similar biophysical properties and enhanced HIV infection rates to similar extents.
Fibrils derived from synthetic peptides have a remarkable ability to increase HIV infection rates: only 1–3 virions are sufficient for productive infection in the presence of fibrils, but 1000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 virions are required in the absence of the fibrils.10,12 Moreover, physiological concentrations of fibrils of approximately 30–40 μg mL−1 increase the infectious titer of HIV-1 by more than five orders of magnitude.
000 virions are required in the absence of the fibrils.10,12 Moreover, physiological concentrations of fibrils of approximately 30–40 μg mL−1 increase the infectious titer of HIV-1 by more than five orders of magnitude.
3. Structure of semen derived amyloids
The precursor protein of PAP(248–286) and PAP(85–120) is a homodimeric enzyme. In the native state of the protein, the 248–286 segment is located close to the dimer interface and it encompasses two α-helices, which extend between residue K251-R257 and G260-I277. The PAP(85–120) fragment constitutes a part of an α-helix, which extends from V77-F92 and plays a crucial role in dimerization. This helix connects the sixth β-sheet of second subunit through a hydrogen bond between D76 and H112.15The primary structure of PAP(248–286) contains eight basic amino acid residues and two acidic amino acid residues (Table 1). These properties result in a theoretical isoelectric point (pI) of 10.21 (Table 1) and a strongly positive net charge at neural pH. Analysis of freshly dissolved PAP(248–286) peptide at 37 °C in 20 mM potassium phosphate buffer, pH 7.5, containing 10% D2O with 1H nuclear magnetic resonance (NMR) spectroscopy revealed a poor dispersion of the chemical shifts within the amide proton region. The recorded spectra lacked nuclear Overhauser effects (NOEs) that would have indicated strong intramolecular interactions or a stable secondary structure.16 Instead, the NMR characteristics suggested that the monomeric peptide was significantly unfolded in aqueous solution but adopted a significantly α-helical conformation in the presence of 20 mM phosphate buffer, pH 7.5, containing 30 or 50% trifluoroethanol (TFE).171H NMR spectroscopy revealed under these conditions strong intramolecular NOEs at 25 °C and the recorded spectra were significantly dispersed. The α-helical segment ranged in 50% TFE from residue Q259 to I284.17 Evidence for an α-helical conformation of PAP(248–286) was also provided by far-ultraviolet (UV) circular dichroism (CD) spectroscopic analysis of a sample containing lipid vesicles formed from 70% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine and 30% 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoglycerol.16 Under these conditions, the α-helical structure identified by NMR extended between residues H250-Q259 and L263-A274.16,18 Hence, binding to these lipid vesicles induced a conformation that is reminiscent of the native conformation of PAP.15
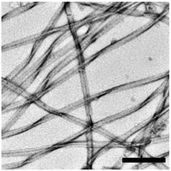
Overnight incubation of PAP(248–286) peptide in Dulbecco's phosphate buffer saline, pH 7.4, at 37 °C led to the aggregation of the peptide and the formation of fibrils that were visible by transmission electron microscopy (TEM) (Fig. 1).10,19 Analysis of these in vitro formed fibrils with atomic force microscopy (AFM) revealed a height of 5.3 ± 0.01 nm and length up to 5 to 10 μm.12 Far-UV CD spectroscopy revealed high levels of β-sheet conformation.20 X-ray diffraction recorded discernible reflections at 4.7 and 10.6 Å, corresponding to the regular inter-strand spacing and inter-sheet distances in aggregated β-sheets.10 The fibrils interacted with the amyloid-binding dyes thioflavin T (ThT) and Congo red (CR) and displayed, taken together with the above characteristics, key features of amyloid fibril structures.10,20 Hydrogen–deuterium exchange coupled with mass spectrometry (HDX-MS) as well as a protease protection assay suggested that an N-terminal segment of aggregated PAP(248–286) comprising residues G248 to E254 was unprotected from HDX and susceptible to proteolytic cleavage. By contrast, a region from K281 to Y286 was found to be highly protected from exchange suggesting that it was involved in formation of fibril core.21 Prediction of highly amyloidogenic sequence elements within PAP(248–286) identified a segment extending between residues G260 to K265 (GGVLVN), which formed microcrystals consisting of self-complementary cross-β sheets, termed steric-zippers.22 This structure allowed the design of a D-amino acid peptide that blocked fibrillation and HIV-1 infectivity enhancement of this peptide (see Section 10.3.3.).
Similar to PAP(248–286), also PAP(85–120) shows a positive net charge at neutral pH and a theoretical pI of 9.99 (Table 1). The peptide formed amyloid fibrils in vitro and displayed ThT and CR binding.12 PAP(85–120) aggregates showed substantial morphological heterogeneity ranging from small spherical oligomers of a height of 3.58 ± 1.22 nm and protofibril-like structures of a height of 4.01 ± 1.23 nm to 1 to 5 μm-long fibrils as determined by AFM.12
The precursor protein of SEM amyloids are the two homologous proteins SEM1 and 2 that are mainly expressed in seminal vesicles and form the major component of semen coagulum.13,14 SEMs are rapidly cleaved after ejaculation by intrinsic proteases and release a number of short peptide fragments, such as SEM1(45–107), SEM2(45–107), SEM1(49–107), SEM2(49–107), SEM1(68–107), SEM2(68–107), and SEM1(86-107).13,14,23 Overnight agitation of these peptides in phosphate buffered saline at 37 °C led to the formation of fibrils as indicated by an increased ThT fluorescence intensity and detection of fibrillar structures by TEM. Under in vitro conditions all the fibrils were capable to enhance HIV infectivity, albeit with different efficiencies.13,14 Based on HDX-MS aggregated SEM1(86–107) seemed to contain a solvent-protected fibril core, including residues D86-K92, S96-R98 and Q104-L107. The positively charged residues L92, L95, A98 were more solvent exposed,23 suggesting that they might be involved in mediating the electrostatic interactions between SEM amyloids and viral particles or host cells.
While atomic structures of in vitro formed fibrils derived from PAP, SEM1 or SEM2 fragments are not yet available, even less is known about the structures of endogenous amyloid fibrils in human semen. Based on AFM and TEM the existence of fibrils in semen has been confirmed (Fig. 2).19,24 Some of the fibrils in semen interacted with the amyloid-binding antibodies WO1 and WO2 and could be immunogold-labelled with primary antibodies binding to PAP- or SEM-derived epitopes.19 These fibrils had diameters of approximately 5–10 nm and showed positive interactions with the amyloid-binding antibodies WO1 and OC.13,19 Notably, seminal amyloid was detected in all ejaculates derived from healthy individuals or HIV-1 infected men, establishing semen as first human body fluid that naturally contains amyloid fibrils in the absence of disease.19
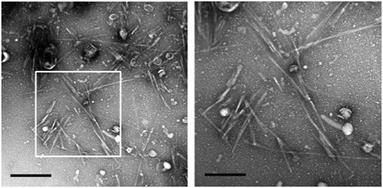 | ||
Fig. 2 TEM of a semen sample. Semen from healthy donor contains amyloid fibrils. Scale bar in left panel represents 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) nm, in right panel 200 nm. Reprinted from ref. 19 with permission from Springer Nature: Nature communications “Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen.” S. M. Usmani, et al., Copyright (2014). nm, in right panel 200 nm. Reprinted from ref. 19 with permission from Springer Nature: Nature communications “Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen.” S. M. Usmani, et al., Copyright (2014). | ||
4. Endogenous semen amyloids and effect of semen on HIV infection
If fibrils are present in semen and contribute to sexual transmission of HIV, semen itself is expected to exert HIV enhancing activity as well. Until the discovery of SEVI fibrils in 2007, however, only a few studies had examined the effects of semen on HIV infection, likely because this biological fluid is quite toxic to cells in vitro,25,26 making it difficult to work with in cell culture-based assays. Since these cytotoxic effects complicate evaluation of semen in vitro, it is critical to study semen under conditions that preserve the viability of target cells.10,27 Under these conditions, which can be achieved limiting the amount of semen cells are exposed to, semen efficiently promotes viral infection.12–14,19,24,28–33 Importantly, the infection-enhancing effects of semen are most prominent at low viral inocula, which to some extent mimics the in vivo situation since only low amounts of virus are present in semen.10,13 As a result, the HIV-promoting effects cannot be observed in systems that require high viral inocula, such as most explant systems.35,36 When low viral doses are used, semen enhances infection of cell types relevant for sexual transmission, including macrophages,27 T cells isolated from cervical and endometrial tissues,10,13 and ex vivo infected vaginal explants.37 This enhancement occurs independently of the HIV-1 strain but still requires the presence of HIV-1 entry receptors CD4 and CXCR4/CCR5.10,27 Semen-mediated enhancement of HIV-1 infection is also observed under acidic pH conditions and in the presence of vaginal fluid.27That the fibrils are important contributors to the ability of semen to enhance HIV infection is supported by three observations. First, donor-dependent variability in the extent of semen-mediated enhancement of HIV infection correlates with the levels of amyloidogenic SEVI and SEM peptides, as measured by ELISA and quantitative mass spectrometry.13,14,27 Second, semen lacking these peptides as a consequence of a congenital condition termed ejaculatory duct obstruction cannot enhance HIV infection.13 Third, extension of the period of semen liquefaction results in progressive loss of the amyloidogenic SEM peptide in a manner that parallels the progressive loss of the infection-enhancing activity of semen.14 However, because semen retains significant infection-enhancing activity for more than 8 h after emission, it has ample opportunity to enhance viral transmission in vivo, which can occur within an hour after exposure in animal models.38 Furthermore, the detection of amyloidogenic SEM peptides in seminal vesicles13,39 suggests that amyloid seeds are already present even before ejaculation, consistent with the observation that fresh ejaculates contain amyloid fibrils and have a potent ability to enhance HIV infection.10,19 Therefore, the “window of opportunity” for semen fibril–mediated enhancement of HIV infection ranges from the time of semen deposition to >8 h later.
5. Mechanism of infection enhancement
Both classes of semen fibrils are derived from peptides with high isoelectric points (Table 1), and only the fibrillar but not the monomeric freshly dissolved peptides enhance viral infection.10,12–14 Zeta potential measurements confirmed that polymerized PAP and SEM fibrils are all highly cationic at neutral pH (Table 1). These properties enable the fibrils to bind to the negatively charged membranes of both HIV virions and cells, which leads to increased viral attachment and fusion to cellular targets.10,12,13 Indeed, abrogating the cationic properties of the fibrils through anionic polymers or site-directed mutagenesis largely diminished their ability to enhance infection.12,14,40 Thus, PAP and SEM derived fibrils enhance viral infection by forming an electrostatic bridge that overcomes the negative repulsions between the viral and cellular membrane, which is normally a rate-limiting step in viral infection.41 However, this mechanism might be an over-simplification as unpublished data by the Fändrich/Münch group suggest that fibrils with negative net surface charges may also capture virions and enhance infection, albeit with lower efficiency than the cationic fibrils in semen.One intriguing question is whether amyloid fibrils in semen may also enhance infection of other viruses besides HIV-1. In fact, it was demonstrated that SEVI fibrils increase infection of human immunodeficiency virus type 2 (HIV-2), a mainly sexually transmitted virus prevalent in Western Africa.27 Similarly, SEVI and SP from humans boosted infection of the closely related simian immunodeficiency virus from macaques (SIVmac).27,33,42 Whether macaque SP may also enhance SIVmac infection could not be analysed due to difficulties in collecting this body fluid from monkeys and the rapid coagulation of macaque semen. However, Zhou et al. demonstrated that synthetic SEVI fibrils derived from the macaque sequence (that differs in one amino acid residue at position 277) enhances infection of HIV-1 and SIV.42 In addition, SEVI and SEM fibrils as well as SP all enhance infection by herpes simplex virus type 1 and 2 (HSV-1 and -2) and cytomegalovirus (CMV).43,44 Addition of the polyanion heparin abolished the HSV/CMV infection-enhancing effects of SEVI, suggesting that the mechanism underlying HSV and CMV infectivity enhancement is similar to that of HIV-1. These findings suggest that seminal amyloids or semen/SP may represent general enhancers of enveloped virus infection.
However, recent findings by Müller et al. show that SEVI fibrils do not affect the infectivity of Zika and Dengue virus, two members of the Flaviviridae family, which are only sparsely transmitted via sexual contact (Müller, Münch et al., accepted). These findings suggest that seminal amyloids do not interact with the flavivirus particle, which could be explained by the observation that the Zika virion is covered by a dense coat of the viral E protein that renders the viral lipid membrane largely inaccessible to large external factors such as seminal fibrils.45 In contrast, the membrane of HIV-1 is largely accessible, because only 5–15 viral glycoprotein spikes are embedded46,47 that may allow efficient interaction of fibrils with HIV-1 particles. Further studies with viruses containing well-defined numbers of viral glycoproteins are needed to clarify whether the accessibility of the viral membrane determines fibril-mediated enhancement of viral infection. In addition, the effect of seminal amyloids on other emerging and re-emerging viruses should be determined as a way to assess the potential of these viruses to be transmitted via sexual intercourse.
The broad and potent ability of seminal amyloid to enhance enveloped virus infection suggests that they might also promote transduction by retro- or lentiviral vectors, which could have practical applications in vitro or in vivo in the context of gene therapy. Indeed, SEVI fibrils also increased infection by a γ-retrovirus48 as well as by retro- and lentiviral vectors.49,50 However, utilization of SEVI as laboratory tool to optimize viral gene transfer is limited because the polypeptide monomers are relatively expensive to produce. These obstacles can be overcome by recently-identified smaller peptides that spontaneously self-assemble into nanofibrils.41,51 These nanofibrils increase retroviral gene transfer even more efficiently than SEVI, are easy to produce and to handle, and are safe as assessed in an ex vivo gene transfer study,51 and have been commercialized as Protransduzin®.
6. Effects on HIV transmission in vivo
Experiments in humanized mice and non-human primates (NHPs) have been used to try to dissect the role of both semen and semen amyloids on lentiviral transmission in vivo. Humanized mice studies have not found a role for semen52 or SEVI53 in affecting HIV transmission rates. However, the difficulty in linking these results to the effects of semen or SEVI on HIV transmission in people is that humanized mouse studies require HIV inocula that are orders of magnitude higher than that naturally present in vivo, which becomes problematic because both semen and SEVI enhance HIV infection only under low, physiologically relevant levels of virus.10 Indeed, in the humanized mouse study characterizing the effects of semen, 5/6 mice were infected in the control group receiving HIV without semen;52 under these conditions enhancement cannot be readily assessed since the vast majority of mice were already infected in the absence of semen. Similarly, in the study assessing the effects of SEVI, large amounts of HIV were used resulting in high baseline transmission rates of 50%.53 Although a lower viral dose was used in one experimental group, one could not compare infection rates between mock- and SEVI-treated groups due to the lack of a mock-treated HIV control.To date, only three studies have examined the effect of SP on lentiviral transmission in NHPs. Two early studies examined the effect of SP on vaginal infection of rhesus macaques by SIV.54,55 Both reported increased vaginal SIV infection in the presence of SP, but only under conditions of low viral inocula. A more recent rhesus macaque vaginal SIV infection study similarly found that SP may facilitate transmission at low viral doses, and further revealed that animals infected in the presence of SP exhibited 6.9-fold higher peak viral loads.33 These results are consistent with the notion that SP and SEVI enhance viral infection most potently under conditions of limiting viral inoculum. Unfortunately, in all three of these macaque studies, animals within the experimental group differed strongly in their susceptibility to vaginal SIV infection. For example, in the most recent study the total viral doses required to establish infection in the control group of animals ranged from 5300 to >128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 Tissue Culture Infection Doses 50 (TCID50) and in the SP group from 100 to >128
500 Tissue Culture Infection Doses 50 (TCID50) and in the SP group from 100 to >128![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 TCID50. As such, the effect of SP on HIV transmission in the NHP system remains unclear. All prior studies have suffered from insufficient statistical power due to a lack of consistency in susceptibility between individual animals within a study group. It should also be noted that SP enhances SIV infection markedly less than HIV-1 infection,33 which blunts the ability of SIV-based studies to assess the infection-promoting effects of SP on HIV in vivo. To this end, the effects of SP should be tested using SHIVs which carry HIV-1 T/F envelopes and can be mucosal transmitted to macaques.56 Such studies should incorporate dose escalation starting with low levels of virus reminiscent of the amount present in the ejaculate of a typical viremic individual. In the absence of such a study, it remains unknown what effect semen is likely to have on HIV transmission in people.
500 TCID50. As such, the effect of SP on HIV transmission in the NHP system remains unclear. All prior studies have suffered from insufficient statistical power due to a lack of consistency in susceptibility between individual animals within a study group. It should also be noted that SP enhances SIV infection markedly less than HIV-1 infection,33 which blunts the ability of SIV-based studies to assess the infection-promoting effects of SP on HIV in vivo. To this end, the effects of SP should be tested using SHIVs which carry HIV-1 T/F envelopes and can be mucosal transmitted to macaques.56 Such studies should incorporate dose escalation starting with low levels of virus reminiscent of the amount present in the ejaculate of a typical viremic individual. In the absence of such a study, it remains unknown what effect semen is likely to have on HIV transmission in people.
7. Role of semen amyloids in reproduction
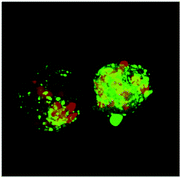
As discussed earlier, SP can have protective effects for sperm and can promote reproductive success through inducing responses in the FRT. What role, if any, do semen amyloids play in these processes? That semen amyloids should play a beneficial role in reproductive success seems likely since the amyloidogenic potential of orthologous peptides is conserved amongst the great apes.14 Should harbouring these amyloid-forming peptides not confer a species survival advantage, they would have presumably been eliminated over evolutionary time due to their ability to enhance infection by HIV and the other sexually transmitted viruses discussed above. Indeed, a recent study suggests that semen amyloids may play a role in sperm selection and clearance. Single-cell analyses by immunofluorescence and electron microscopy revealed that the membranes of sperm cells interact directly with semen amyloid fibrils.32 This observation, together with the knowledge that semen elicits a leukocytic response characterized by infiltration of neutrophils and macrophages into the lower FRT,57 led to the hypothesis that entrapment of sperm cells by semen amyloids may promote their engulfment by infiltrating phagocytes. This was shown experimentally by demonstrating the ability of semen amyloids to promote phagocytosis of sperm cells by macrophages.32 Strikingly, in the presence of amyloids, individual macrophages were seen to engulf up to over a dozen sperm heads (Fig. 3). The rapid removal of sperm cells from the lower FRT may help ensure that no inappropriate immune response is developed against male antigens, and is consistent with the observation that the lower FRT largely returns to its pre-mating state within 24 hours.57 On the other hand, if semen amyloids promote sperm disposal in the lower FRT, how do spermatozoa make it to the oviduct? Intriguingly, macrophages have the ability to preferentially engulf damaged or dead sperm over live motile ones, and semen amyloids increased the efficiency of this mechanism.32 Therefore, semen amyloids may promote the efficiency of sperm selection by phagocytes infiltrating the lower FRT, ensuring the rapid removal of damaged sperm and lingering sperm antigens, and allowing for only the most robust spermatozoa (a small fraction of the deposited number58) to reach the oviduct isthmus.8. Role of semen amyloids in innate immunity
Findings by Easterhoff et al. suggest that semen fibrils may also exert indirect antibacterial activity upon deposition in the FRT.59 Based on the antimicrobial properties of other amyloidogenic peptides such as amyloid-β (1–42),60 the antimicrobial activity of SEVI was examined. Neither SEVI fibrils nor the unassembled PAP(248–286) peptide displayed any direct antibacterial activity.59 However, similarly to the interaction with eukaryotic cells, SEVI fibrils bound to and entrapped both Gram-positive and Gram-negative bacteria. Similar to the interactions between SEVI and enveloped viruses, sequestration of bacteria occurred in a charge-dependent fashion. This has been proven by the fact that a non-cationic SEVI variant failed to bind bacteria.59 In turn, SEVI-aggregated bacteria were more efficiently phagocytosed by primary human macrophages, which resulted in an elevated release of bacterially induced pro-inflammatory cytokines. Finally, SEVI fibrils inhibited vaginal colonization with Neisseria gonorrhoea in a murine model.59 Interestingly, it has also been demonstrated that bacterial curli proteins promote conversion of PAP(248–286) into SEVI fibrils.61 Since curli fibers from bacteria may colocalize with PAP(248–286) at the initial sites of HIV infection, they may induce and accelerate the formation of antibacterial SEVI fibrils trough a cross-seeding mechanism. Together these results demonstrate that SEVI and perhaps other semen fibrils represent a novel class of immune defense molecules with both immunomodulatory and indirect antimicrobial activity.9. Semen fibrils and microbicide activity against HIV
Anti-HIV microbicides have been sought after as a way to limit worldwide rates of HIV transmission, particularly in resource-limited. Microbicides are compounds to be applied inside the vagina or rectum to protect against sexually transmitted viral infections, including HIV. Although many microbicides have demonstrated potent anti-HIV activity in cell culture, they have largely failed to exert protective effects in clinical trials in humans.62 Zirafi et al. showed that in the presence of SEVI amyloid or semen, most candidate microbicides – including neutralizing antibodies and standard antiretroviral drugs – showed greatly reduced antiviral efficacy.31 This diminished antiviral activity was dependent on the ability of semen fibrils to enhance viral infectivity because semen depleted of amyloid due to prolonged incubation at 37 °C14 or naturally-deficient in amyloid13 did not impair microbicide efficacy.31 The molecular basis of how semen and SEVI diminishes the anti-HIV effects of microbicide candidates is not clear. One explanation is that the fibrils bind to HIV virions and efficiently enhance and accelerate their attachment to target cells,10,13,27,40 thereby shortening the time of virion exposure to neutralizing antibodies. In addition, in the presence of fibrils multiple infections of an individual cell may take place, which results in elevated intracellular levels of viral enzymes, requiring increased concentrations of drugs. Regardless of mechanism, the finding that semen impairs the antiviral efficacy of microbicides has important implications. First, before entering clinical trials, promising microbicide candidates should be tested in multiple assays that incorporate the effects of semen.63 The second implication is that agents that antagonize seminal amyloid may exert dual beneficial effects because they abolish viral infectivity enhancement and restore the antiviral activity of microbicides in the presence of semen.10. Semen amyloid antagonists
Several strategies are conceivable to antagonize the HIV enhancing activity of semen in order to prevent sexual virus transmission and to increase the antiviral activity of microbicides.10,64–67 One possibility is to prevent the formation of mature fibrils in semen through blocking the proteolytic generation of PAP and SEM peptides from its precursor proteins. A second approach is to inhibit the assembly of PAP and SEM fragments into the mature infection-enhancing fibrils. However, since mature fibrils are already present in fresh ejaculates, it seems a more effective strategy to remodel or disassemble preformed fibrils in a way that they lose infection-enhancing activity. Finally, shielding or neutralizing the charged surface of the fibrils should disrupt the ability of the fibrils to promote the interactions between viruses and cells, thereby abolishing infection-enhancement. Several agents have been described in the past 10 years that antagonize seminal amyloid through one or more mechanisms (Table 2). These include metal ions, small molecule compounds, peptides, proteins, polymers and nanoparticles, and will be discussed in the following sections.| Name | Structure/class | Mode of action | Amyloid | SP | Ref. |
|---|---|---|---|---|---|
| n.d., not determined. | |||||
| Cu2+ | Anorganic ion | Assembly blocker | SEVI | n.d. | 68 |
| Zn2+ | Anorganic ion | Assembly blocker | SEVI, SEM1 and SEM1(1–159) | n.d. | 68 and 100 |
| Gallic acid | Small molecule | Neutralization/coating | SEVI, SEM1(86–107) | Yes | 74 |
| Brazilin | Small molecule | Assembly blocker | SEVI | n.d. | 88 |
| Surfen | Small molecule | Neutralization/coating | SEVI | Yes | 30 |
| EGCG | Small molecule | Assembly blocker, remodeling, direct antiviral activity | SEVI, PAP(85–120), SEM1(45–107), and SEM2(49–107) | Yes | 24, 70, 72 and 73 |
| BTA-EG6 | Small molecule | Neutralization/coating | SEVI | Yes | 76 and 101 |
| CLR01 | Small molecule | Assembly blocker, remodeling, neutralization/coating, direct antiviral activity | SEVI, PAP(85–120); SEM1(45–107) | Yes | 29 |
| ADS-J1 | Small molecule | Assembly blocker, neutralization/coating, direct antiviral activity | SEVI | Yes | 77 |
| WW61 | Peptide | Assembly blocker | SEVI | n.d. | 22 |
| D3 | Peptide | Remodeling | SEVI | n.d. | 90 |
| E4(ChaKChaE)2 | Peptide | Neutralization/coating | SEVI | n.d. | 92 |
| BTA oligomers | Polymer | Neutralization/coating | SEVI | n.d. | 69 |
| Hsp104 | Protein | Remodeling | SEVI, PAP(85–120), SEM1(45–107) | n.d. | 94 |
| Polyanions (heparin, dextran sulfate) | Polymer | Neutralization/coating, direct antiviral activity | SEVI | Yes | 12 and 40 |
| Polymeric nanoparticles | Nanoparticle | Neutralization/coating | SEVI | n.d. | 67 |
| Hydrophobic nanoparticles | Nanoparticle | Remodeling | SEVI | n.d. | 99 |
10.1. Metal ions
10.2. Small molecule antagonists
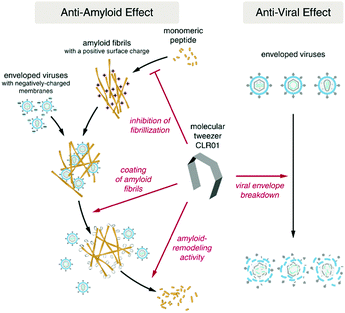 | ||
| Fig. 4 The molecular tweezer CLR01 acts as a dual-function inhibitor of viral infection featuring both anti-amyloid and antiviral activity. Adapted with permissions from ref. 29. | ||
10.3. Peptide-based antagonists
10.4. Proteins
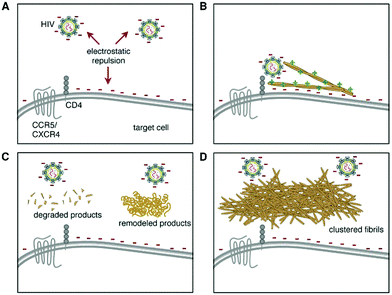 | ||
| Fig. 5 Hsp104-based treatments that remodel, degrade, or cluster seminal amyloid reduce their ability to stimulate HIV Infection. (A) Electrostatic charge repulsion between the negatively charged surfaces of the viral and target cell membranes. (B) Cationic seminal amyloid fibrils act as “electrostatic bridge” and increase viral attachment and infection. (C) Hsp104 remodels and degrades semen fibrils thereby by antagonizing infection enhancement. (D) Hsp104 promotes the assembly of seminal amyloid into higher-order conglomerates with greatly reduced HIV promoting activity. Reprinted from ref. 94, “Repurposing Hsp104 to antagonize seminal amyloid and counter HIV infection.” 22.8, L. M. Castellano, et al., 1074–1086, Copyright (2015), with permission from Elsevier. | ||
10.5. Polymeric structures
10.6. Preclinical and clinical development of semen amyloid antagonists
Although several agents have been shown to counteract the viral-enhancing activity of seminal amyloids in cell culture, none of these have advanced to preclinical development as HIV preventative microbicides. The main reason for why these encouraging in vitro findings have not been translated into new prevention strategies is the lack of an appropriate animal model that allows the field to readily assess the HIV-enhancing effects of semen or seminal amyloid on viral transmission (for details see Section 6). Perhaps even more important is the consideration that most if not all agents that counteract fibril-mediated infection enhancement will likely also interfere with the natural functions of semen amyloids (for details see Sections 7 and 8). Agents that suppress fibril formation, disassemble or remodel fibrils, or form coatings to prevent the interaction with virions and cells, will most likely also abrogate the interaction of fibrils with defective sperm cells32 or bacterial pathogens,59 and hence disturb sperm clearance and antibacterial immune responses in the FRT. Thus, counteracting semen fibrils to block viral transmission may come at the cost of affecting fertility or general reproductive health.11. Open questions
This review summarized the current knowledge on seminal amyloids gained in the past decade. Still several questions regarding the structures and functions of these intriguing components in semen remain unanswered. For example, what is the molecular structure of PAP and SEM fibrils, are there additional types of semen fibrils, and what are the concentrations and relative compositions of amyloids in semen? Where and when are PAP and SEM peptides generated and where and with what kinetics do fibrils form? How is fibrillation regulated? What is the exact mechanism underlying the interaction of fibrils with virions and cells, and what is the fate of the fibrils after delivery of the viral cargo to the cell surface? Do the fibrils have signalling effects in the female reproductive tract? Is aberrant fibril formation or degradation associated with infertility or increased susceptibility to sexually transmitted diseases? Are there other amyloids naturally present in the human body that enhance virus infection and may contribute to viral pathogenesis? Clearly, much about the role of human amyloids in health and disease remains to be worked out.12. Concluding remarks
Semen fibrils are fascinating functional amyloids with unprecedented roles in sperm selection and removal as well as innate immunity. They are present in ejaculates of all men and may be exploited by HIV for effective viral transmission. Since their identification ten years ago, we have learned a lot the structural, functional, and pathogenic effects of these intriguing structures. We suspect the next ten years will yield an additional wealth of information about the role of semen amyloids in reproductive health and viral infections, and whether they can serve as biomarkers for human diseases.Conflicts of interest
J. M. has out-licensed a patent on the utilization of self-assembling peptides to promote lentiviral gene transfer, which is commercialized under the brand name Protransduzin®. Remaining authors have no conflict of interest to declare.Acknowledgements
J. M. and M. F. acknowledge funding from the Deutsche Forschungsgemeinschaft (CRC1279 A03). J. M. receives additional support from the Volkswagen Stiftung, and the Leibniz Gemeinschaft. N. R. R. acknowledges funding by the National Institutes of Health (R21 AI116252 and R21 AI122821). A. R. is funded by a fellowship of the Landesgraduiertenförderung Baden-Württemberg and is part of the International Graduate School in Molecular Medicine Ulm. J. K. Y. acknowledges the funding by the project UULM PRO MINT & MED under the direction of Prof. Dr Irene Bouw, Vice President for Teaching and International Affairs at Ulm University.References
- O. Wai-Sum, H. Chen and P. H. Chow, Male genital tract antioxidant enzymes–their ability to preserve sperm DNA integrity, Mol. Cell. Endocrinol., 2006, 250, 80–83 CrossRef PubMed.
- J. J. Bromfield, Seminal fluid and reproduction: much more than previously thought, J. Assist. Reprod. Genet., 2014, 31, 627–636 CrossRef PubMed.
- G. Crawford, A. Ray, A. Gudi, A. Shah and R. Homburg, The role of seminal plasma for improved outcomes during in vitro fertilization treatment: review of the literature and meta-analysis, Hum. Reprod. Update, 2015, 21, 275–284 CrossRef PubMed.
- D. J. Sharkey, et al., TGF-β mediates proinflammatory seminal fluid signaling in human cervical epithelial cells, J. Immunol., 2012, 189, 1024–1035 CrossRef PubMed.
- D. J. Sharkey, K. P. Tremellen, M. J. Jasper, K. Gemzell-Danielsson and S. A. Robertson, Seminal fluid induces leukocyte recruitment and cytokine and chemokine mRNA expression in the human cervix after coitus, J. Immunol., 2012, 188, 2445–2454 CrossRef PubMed.
- J. C. Chen, et al., Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts, Hum. Reprod., 2014, 29, 1255–1270 CrossRef PubMed.
- L. Vojtech, et al., Exosomes in human semen carry a distinctive repertoire of small non-coding RNAs with potential regulatory functions, Nucleic Acids Res., 2014, 42, 7290–7304 CrossRef PubMed.
- J. Münch, L. Ständker, W.-G. Forssmann and F. Kirchhoff, Discovery of modulators of HIV-1 infection from the human peptidome, Nat. Rev. Microbiol., 2014, 12, 715–722 CrossRef PubMed.
- J. Münch, et al., Discovery and Optimization of a Natural HIV-1 Entry Inhibitor Targeting the gp41 Fusion Peptide, Cell, 2007, 129, 263–275 CrossRef PubMed.
- J. Münch, et al., Semen-Derived Amyloid Fibrils Drastically Enhance HIV Infection, Cell, 2007, 131, 1059–1071 CrossRef PubMed.
- L. Rönnberg, P. Vihko, E. Sajanti and R. Vihko, Clomiphene citrate administration to normogonadotropic subfertile men: blood hormone changes and activation of acid phosphatase in seminal fluid, Int. J. Androl., 1981, 4, 372–378 CrossRef.
- F. Arnold, et al., Naturally Occurring Fragments from Two Distinct Regions of the Prostatic Acid Phosphatase Form Amyloidogenic Enhancers of HIV Infection, J. Virol., 2012, 86, 1244–1249 CrossRef PubMed.
- N. R. Roan, et al., Peptides released by physiological cleavage of semen coagulum proteins form amyloids that enhance HIV infection, Cell Host Microbe, 2011, 10, 541–550 Search PubMed.
- N. R. Roan, et al., Liquefaction of Semen Generates and Later Degrades a Conserved Semenogelin Peptide That Enhances HIV Infection, J. Virol., 2014, 88, 7221–7234 CrossRef PubMed.
- C. G. Jakob, K. Lewinski, R. Kuciel, W. Ostrowski and L. Lebioda, Crystal structure of human prostatic acid phosphatase, Prostate, 2000, 42, 211–218 CrossRef PubMed.
- J. R. Brender, et al., Helical conformation of the SEVI precursor peptide PAP248-286, a dramatic enhancer of HIV infectivity, promotes lipid aggregation and fusion, Biophys. J., 2009, 97, 2474–2483 CrossRef PubMed.
- J. R. Brender, et al., The amyloidogenic SEVI precursor, PAP248-286, is highly unfolded in solution despite an underlying helical tendency, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 1161–1169 CrossRef PubMed.
- R. P. R. Nanga, J. R. Brender, S. Vivekanandan, N. Popovych and A. Ramamoorthy, NMR structure in a membrane environment reveals putative amyloidogenic regions of the SEVI precursor peptide PAP248-286, J. Am. Chem. Soc., 2009, 131, 17972–17979 CrossRef PubMed.
- S. M. Usmani, et al., Direct visualization of HIV-enhancing endogenous amyloid fibrils in human semen, Nat. Commun., 2014, 5, 3508 CrossRef PubMed.
- Z. Ye, et al., Mechanism of fibril formation by a 39-residue peptide (PAPf39) from human prostatic acidic phosphatase, Biochemistry, 2009, 48, 11582–11591 CrossRef PubMed.
- K. C. French and G. I. Makhatadze, Core sequence of papf39 amyloid fibrils and mechanism of ph-dependent fibril formation: The role of monomer conformation, Biochemistry, 2012, 51, 10127–10136 CrossRef PubMed.
- S. A. Sievers, et al., Structure-based design of non-natural amino-acid inhibitors of amyloid fibril formation, Nature, 2011, 475, 96 CrossRef PubMed.
- K. C. French, N. R. Roan and G. I. Makhatadze, Structural characterization of semen coagulum-derived SEM1(86-107) amyloid fibrils that enhance HIV-1 infection, Biochemistry, 2014, 53, 3267–3277 CrossRef PubMed.
- P. Hartjen, et al., Assessment of the range of the HIV-1 infectivity enhancing effect of individual human semen specimen and the range of inhibition by EGCG, AIDS Res. Ther., 2012, 9, 2 CrossRef PubMed.
- R. D. Allen and T. K. Roberts, The relationship between the immunosuppressive and cytotoxic effects of human seminal plasma, Am. J. Reprod. Immunol. Microbiol., 1986, 11, 59–64 CrossRef.
- R. D. Allen and T. K. Roberts, The role of spermine in the cytotoxic effects of seminal plasma, J. Immunol., 1987, 138, 656 Search PubMed.
- K. A. Kim, et al., Semen-mediated enhancement of HIV infection is donor-dependent and correlates with the levels of SEVI, Retrovirology, 2010, 7, 55 CrossRef PubMed.
- C. Camus, et al., Comparison of the effect of semen from HIV-infected and uninfected men on CD4+T-cell infection, AIDS, 2016, 30, 1197 CrossRef PubMed.
- E. Lump, et al., A molecular tweezer antagonizes seminal amyloids and HIV infection, eLife, 2015, 4 DOI:10.7554/eLife.05397.
- N. R. Roan, S. Sowinski, J. Münch, F. Kirchhoff and W. C. Greene, Aminoquinoline surfen inhibits the action of SEVI (Semen-derived Enhancer of Viral Infection), J. Biol. Chem., 2010, 285, 1861–1869 CrossRef PubMed.
- O. Zirafi, et al., Semen enhances HIV infectivity and impairs the antiviral efficacy of microbicides, Sci. Transl. Med., 2014, 6, 262ra157 CrossRef PubMed.
- N. R. Roan, et al., Semen amyloids participate in spermatozoa selection and clearance, eLife, 2017, 6 DOI:10.7554/eLife.24888.
- J. Münch, et al., Effect of semen and seminal amyloid on vaginal transmission of simian immunodeficiency virus, Retrovirology, 2013, 10, 148 CrossRef PubMed.
- F. Yu, et al., ADS-J1 inhibits HIV-1 infection and membrane fusion by targeting the highly conserved pocket in the gp41 NHR-trimer, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 1296–1305 CrossRef PubMed.
- S. A. Allen, et al., Characterization of the Influence of Semen-Derived Enhancer of Virus Infection on the Interaction of HIV-1 with Female Reproductive Tract Tissues, J. Virol., 2015, 89, 5569–5580 CrossRef PubMed.
- K. Kordy, et al., Human semen or seminal plasma do not enhance HIV-1Bal ex vivo infection of human colonic explants, AIDS Res. Hum. Retroviruses, 2018 DOI:10.1089/aid.2017.0118.
- A. Introini, et al., Seminal plasma induces inflammation and enhances HIV-1 replication in human cervical tissue explants, PLoS Pathog., 2017, 13, e1006402 Search PubMed.
- J. Hu, M. B. Gardner and C. J. Miller, Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells, J. Virol., 2000, 74, 6087–6095 CrossRef PubMed.
- R. P. Linke, et al., Senile seminal vesicle amyloid is derived from semenogelin I, J. Lab. Clin. Med., 2005, 145, 187–193 CrossRef PubMed.
- N. R. Roan, et al., The Cationic Properties of SEVI Underlie Its Ability To Enhance Human Immunodeficiency Virus Infection, J. Virol., 2009, 83, 73–80 CrossRef PubMed.
- C. Meier, T. Weil, F. Kirchhoff and J. Münch, Peptide nanofibrils as enhancers of retroviral gene transfer, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2014, 6, 438–451 CrossRef PubMed.
- R. H. Zhou, et al., Epigallocatechin Gallate Inhibits Macaque SEVI-Mediated Enhancement of SIV or SHIV Infection, JAIDS, J. Acquired Immune Defic. Syndr., 2017, 75, 232–240 CrossRef PubMed.
- Q. Tang, N. R. Roan and Y. Yamamura, Seminal Plasma and Semen Amyloids Enhance Cytomegalovirus Infection in Cell Culture, J. Virol., 2013, 87, 12583–12591 CrossRef PubMed.
- L. Torres, T. Ortiz and Q. Tang, Enhancement of herpes simplex virus (HSV) infection by seminal plasma and semen amyloids implicates a new target for the prevention of HSV infection, Viruses, 2015, 7, 2057–2073 CrossRef PubMed.
- V. A. Kostyuchenko, et al., Structure of the thermally stable Zika virus, Nature, 2016, 533, 425–428 CrossRef PubMed.
- E. Chertova, et al., Envelope glycoprotein incorporation, not shedding of surface envelope glycoprotein (gp120/SU), Is the primary determinant of SU content of purified human immunodeficiency virus type 1 and simian immunodeficiency virus, J. Virol., 2002, 76, 5315–5325 CrossRef PubMed.
- J. Chojnacki, et al., Maturation-Dependent HIV-1 Surface Protein Redistribution Revealed by Fluorescence Nanoscopy, Science, 2012, 338, 524–528 CrossRef PubMed.
- S. Hong, et al., Fibrils of prostatic acid phosphatase fragments boost infections with XMRV (xenotropic murine leukemia virus-related virus), a human retrovirus associated with prostate cancer, J. Virol., 2009, 83, 6995–7003 CrossRef PubMed.
- M. Wurm, et al., The influence of semen-derived enhancer of virus infection on the efficiency of retroviral gene transfer, J. Gene Med., 2010, 12, 137–146 Search PubMed.
- M. Wurm, et al., Improved lentiviral gene transfer into human embryonic stem cells grown in co-culture with murine feeder and stroma cells, Biol. Chem., 2011, 392, 887–895 CrossRef PubMed.
- M. Yolamanova, et al., Peptide nanofibrils boost retroviral gene transfer and provide a rapid means for concentrating viruses, Nat. Nanotechnol., 2013, 8, 130 CrossRef PubMed.
- O. D. Council, M. D. Swanson, R. A. Spagnuolo, A. Wahl and J. V. Garcia, Role of semen on vaginal HIV-1 transmission and maraviroc protection, Antimicrob. Agents Chemother., 2015, 59, 7847–7851 CrossRef PubMed.
- E. S. V. Dis, et al., No SEVI-mediated enhancement of rectal HIV-1 transmission of HIV-1 in two humanized mouse cohorts, Virology, 2016, 488, 88–95 CrossRef PubMed.
- C. J. Miller, et al., Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques, Lab. Invest., 1994, 70, 255–262 Search PubMed.
- O. Neildez, et al., Variation in virological parameters and antibody responses in macaques after atraumatic vaginal exposure to a pathogenic primary isolate of SIVmac251, Res. Virol., 1998, 149, 53–68 CrossRef PubMed.
- G. Q. Del Prete, et al., Selection of unadapted, pathogenic SHIVs encoding newly transmitted HIV-1 envelope proteins, Cell Host Microbe, 2014, 16, 412–418 Search PubMed.
- I. J. Pandya and J. Cohen, The leukocytic reaction of the human uterine cervix to spermatozoa, Fertil. Steril., 1985, 43, 417–421 CrossRef PubMed.
- M. Eisenbach and L. C. Giojalas, Sperm guidance in mammals—an unpaved road to the egg, Nat. Rev. Mol. Cell Biol., 2006, 7, 276–285 CrossRef PubMed.
- D. Easterhoff, et al., Semen-derived enhancer of viral infection (SEVI) binds bacteria, enhances bacterial phagocytosis by macrophages, and can protect against vaginal infection by a sexually transmitted bacterial pathogen, Antimicrob. Agents Chemother., 2013, 57, 2443–2450 CrossRef PubMed.
- D. K. V. Kumar, et al., Amyloid-β peptide protects against microbial infection in mouse and worm models of Alzheimer's disease, Sci. Transl. Med., 2016, 8, 340ra72 CrossRef PubMed.
- K. Hartman, et al., Bacterial curli protein promotes the conversion of PAP 248-286 into the amyloid SEVI: cross-seeding of dissimilar amyloid sequences, PeerJ, 2013, 1, e5 Search PubMed.
- K. B. Alexandre, H. T. Mufhandu, G. M. London, E. Chakauya and M. Khati, Progress and Perspectives on HIV-1 microbicide development, Virology, 2016, 497, 69–80 CrossRef PubMed.
- N. R. Roan and J. Münch, Improving preclinical models of HIV microbicide efficacy, Trends Microbiol., 2015, 23, 445–447 CrossRef PubMed.
- F. Kirchhoff and J. Münch, Blocking semen-mediated enhancement of HIV infection by amyloid-binding small molecules, Future Virol., 2011, 6, 183–186 CrossRef.
- L. M. Castellano and J. Shorter, The Surprising Role of Amyloid Fibrils in HIV Infection, Biology, 2012, 1, 58–80 CrossRef PubMed.
- N. R. Roan and W. C. Greene, A Seminal Finding for Understanding HIV Transmission, Cell, 2007, 131, 1044–1046 CrossRef PubMed.
- D. A. Sheik, L. Brooks, K. Frantzen, S. Dewhurst and J. Yang, Inhibition of the enhancement of infection of human immunodeficiency virus by semen-derived enhancer of virus infection using amyloid-targeting polymeric nanoparticles, ACS Nano, 2015, 9, 1829–1836 CrossRef PubMed.
- S. R. Sheftic, J. M. Snell, S. Jha and A. T. Alexandrescu, Inhibition of semen-derived enhancer of virus infection (SEVI) fibrillogenesis by zinc and copper, Eur. Biophys. J., 2012, 41, 695–704 CrossRef PubMed.
- C. C. Capule, C. Brown, J. S. Olsen, S. Dewhurst and J. Yang, Oligovalent amyloid-binding agents reduce SEVI-mediated enhancement of HIV-1 infection, J. Am. Chem. Soc., 2012, 134, 905–908 CrossRef PubMed.
- I. Hauber, H. Hohenberg, B. Holstermann, W. Hunstein and J. Hauber, The main green tea polyphenol epigallocatechin-3-gallate counteracts semen-mediated enhancement of HIV infection, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 9033–9038 CrossRef PubMed.
- T. Kada, K. Kaneko, S. Matsuzaki, T. Matsuzaki and Y. Hara, Detection and chemical identification of natural bio-antimutagens. A case of the green tea factor, Mutat. Res., 1985, 150, 127–132 CrossRef PubMed.
- N. Popovych, et al., Site specific interaction of the polyphenol EGCG with the SEVI amyloid precursor peptide PAP(248-286), J. Phys. Chem. B, 2012, 116, 3650–3658 CrossRef PubMed.
- L. M. Castellano, R. M. Hammond, V. M. Holmes, D. Weissman and J. Shorter, Epigallocatechin-3-gallate rapidly remodels PAP85-120, SEM1(45-107), and SEM2(49-107) seminal amyloid fibrils, Biol. Open, 2015, 4, 1206–1212 CrossRef PubMed.
- J. G. LoRicco, et al., Gallic Acid Is an Antagonist of Semen Amyloid Fibrils That Enhance HIV-1 Infection, J. Biol. Chem., 2016, 291, 14045–14055 CrossRef PubMed.
- P. Inbar, C. Q. Li, S. A. Takayama, M. R. Bautista and J. Yang, Oligo(ethylene glycol) derivatives of thioflavin T as inhibitors of protein-amyloid interactions, ChemBioChem, 2006, 7, 1563–1566 CrossRef PubMed.
- J. S. Olsen, et al., Amyloid-binding small molecules efficiently block SEVI (Semen-derived Enhancer of Virus Infection)- and semen-mediated enhancement of HIV-1 infection, J. Biol. Chem., 2010, 285, 35488–35496 CrossRef PubMed.
- T. Xun, et al., ADS-J1 inhibits semen-derived amyloid fibril formation and blocks fibril-mediated enhancement of HIV-1 infection, Antimicrob. Agents Chemother., 2015, 59, 5123–5134 CrossRef PubMed.
- S. Tan, et al., Polyanionic Candidate Microbicides Accelerate the Formation of Semen-Derived Amyloid Fibrils to Enhance HIV-1 Infection, PLoS One, 2013, 8, e59777 Search PubMed.
- D. H. J. Lopes, et al., Molecular tweezers inhibit islet amyloid polypeptide assembly and toxicity by a new mechanism, ACS Chem. Biol., 2015, 10, 1555–1569 CrossRef PubMed.
- S. Sinha, et al., Lysine-specific molecular tweezers are broad-spectrum inhibitors of assembly and toxicity of amyloid proteins, J. Am. Chem. Soc., 2011, 133, 16958–16969 CrossRef PubMed.
- X. Zheng, et al., Amyloid β-protein assembly: The effect of molecular tweezers CLR01 and CLR03, J. Phys. Chem. B, 2015, 119, 4831–4841 CrossRef PubMed.
- A. J. Doig and P. Derreumaux, Inhibition of protein aggregation and amyloid formation by small molecules, Curr. Opin. Struct. Biol., 2015, 30, 50–56 CrossRef PubMed.
- S. Prabhudesai, et al., A novel molecular tweezer inhibitor of α-synuclein neurotoxicity in vitro and in vivo, Neurotherapeutics, 2012, 9, 464–476 CrossRef PubMed.
- A. Attar, et al., Protection of primary neurons and mouse brain from Alzheimer's pathology by molecular tweezers, Brain, 2012, 135, 3735–3748 CrossRef PubMed.
- F. Richter, et al., A Molecular Tweezer Ameliorates Motor Deficits in Mice Overexpressing α-Synuclein, Neurotherapeutics, 2017, 14, 1107–1119 CrossRef PubMed.
- A. Attar, W.-T. C. Chan, F.-G. Klärner, T. Schrader and G. Bitan, Safety and pharmacological characterization of the molecular tweezer CLR01 – a broad-spectrum inhibitor of amyloid proteins’ toxicity, BMC Pharmacol. Toxicol., 2014, 15, 23 CrossRef PubMed.
- W.-J. Du, et al., Brazilin inhibits amyloid β-protein fibrillogenesis, remodels amyloid fibrils and reduces amyloid cytotoxicity, Sci. Rep., 2015, 5, 7992 CrossRef PubMed.
- M. Li, X. Dong, Y. Liu and Y. Sun, Brazilin Inhibits Prostatic Acidic Phosphatase Fibrillogenesis and Decreases its Cytotoxicity, Chem. – Asian J., 2017, 12, 1062–1068 CrossRef PubMed.
- S. Aileen Funke, et al., Oral treatment with the d-enantiomeric peptide D3 improves the pathology and behavior of Alzheimer's Disease transgenic mice, ACS Chem. Neurosci., 2010, 1, 639–648 CrossRef PubMed.
- M. Widera, et al., The D-amino acid peptide D3 reduces amyloid fibril boosted HIV-1 infectivity, AIDS Res. Ther., 2014, 11, 1 CrossRef PubMed.
- W. M. Wojtowicz, et al., Stimulation of Enveloped Virus Infection by β-Amyloid Fibrils, J. Biol. Chem., 2002, 277, 35019–35024 CrossRef PubMed.
- D. Easterhoff, J. T. M. DiMaio, T. M. Doran, S. Dewhurst and B. L. Nilsson, Enhancement of HIV-1 infectivity by simple, self-assembling modular peptides, Biophys. J., 2011, 100, 1325–1334 CrossRef PubMed.
- K. L. Mack and J. Shorter, Engineering and Evolution of Molecular Chaperones and Protein Disaggregases with Enhanced Activity, Front. Mol. Biosci., 2016, 3, 8 Search PubMed.
- L. M. Castellano, S. M. Bart, V. M. Holmes, D. Weissman and J. Shorter, Repurposing Hsp104 to Antagonize Seminal Amyloid and Counter HIV Infection, Chem. Biol., 2015, 22, 1074–1086 CrossRef PubMed.
- L. N. Callahan, M. Phelan, M. Mallinson and M. A. Norcross, Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions, J. Virol., 1991, 65, 1543–1550 Search PubMed.
- C. C. Rider, The potential for heparin and its derivatives in the therapy and prevention of HIV-1 infection, Glycoconjugate J., 1997, 14, 639–642 CrossRef PubMed.
- S. Skoler-Karpoff, et al., Efficacy of Carraguard for prevention of HIV infection in women in South Africa: a randomised, double-blind, placebo-controlled trial, Lancet, 2008, 372, 1977–1987 CrossRef.
- S. McCormack, et al., PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial, Lancet, 2010, 376, 1329–1337 CrossRef.
- D. A. Sheik, et al., Hydrophobic Nanoparticles Reduce the β-Sheet Content of SEVI Amyloid Fibrils and Inhibit SEVI-Enhanced HIV Infectivity, Langmuir, 2017, 33, 2596–2602 CrossRef PubMed.
- N. Sharma and B. K. Patel, Recombinant Human Semenogelin-1 (Sg1) and Sg1 (1-159) form Detergent Stable Amyloid Like Aggregates In Vitro, Protein Pept. Lett., 2016, 23, 87–96 CrossRef PubMed.
- D. A. Sheik, S. Dewhurst and J. Yang, Natural Seminal Amyloids as Targets for Development of Synthetic Inhibitors of HIV Transmission, Acc. Chem. Res., 2017, 50, 2159–2166 CrossRef PubMed.
| This journal is © The Royal Society of Chemistry 2018 |