Multitude NMR studies of α-synuclein familial mutants: probing their differential aggregation propensities†
Abstract
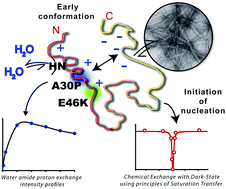
Familial mutations in α-synuclein affect the immediate chemical environment of the protein's backbone, changing its aggregation kinetics and forming diverse structural and functional intermediates. This study, concerning two oppositely aggregating mutants A30P and E46K, reveals a completely diverse conformational landscape for each, thus providing atomistic insights into differences in their aggregation dynamics.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...