Role of the carboxy groups of triterpenoids in their inhibition of the nucleation of amyloid β42 required for forming toxic oligomers†
Abstract
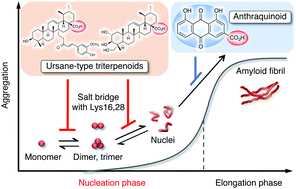
Herein we report that a preferable inhibition of the nucleation phase of Aβ42, related to the formation of toxic oligomers, by triterpenoids from medicinal herbs originates from a salt bridge of their carboxy groups with Lys16 and 28 in Aβ42. Such a direct interaction targeting the monomer, dimer, and trimer suppressed further oligomerization. In contrast, the corresponding congeners without carboxy groups failed to do so.
- This article is part of the themed collection: Amyloid Aggregation


 Please wait while we load your content...
Please wait while we load your content...