Themed collection Enzyme catalysis in organic synthesis

Enzyme catalysis in organic synthesis
Editorial to accompany the Green Chemistry themed issue “Enzyme Catalysis in Organic Chemistry”.
Green Chem., 2017,19, 331-332
https://doi.org/10.1039/C6GC90124G
Transaminase biocatalysis: optimization and application
Transaminase biocatalysis shows immense potential in industrial applications, and optimizations of both proteins and processes are of great importance.
Green Chem., 2017,19, 333-360
https://doi.org/10.1039/C6GC02328B
One-step asymmetric synthesis of (R)- and (S)-rasagiline by reductive amination applying imine reductases
Imine reductases (IREDs) show great potential as catalysts for reductive amination of ketones to produce chiral secondary amines.
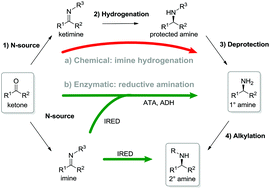
Green Chem., 2017,19, 385-389
https://doi.org/10.1039/C6GC03023H
Asymmetric synthesis of (S)-phenylacetylcarbinol – closing a gap in C–C bond formation
By the combination of biocatalyst design and reaction engineering, the so far not stereoselectively accessible (S)-phenylacetylcarbinol could be enzymatically synthesized with product concentrations >48 g L−1 and an enantiomeric excess up to 97%.
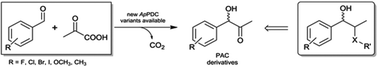
Green Chem., 2017,19, 380-384
https://doi.org/10.1039/C6GC01803C
Photobiocatalytic alcohol oxidation using LED light sources
The photocatalytic oxidation of NADH using a flavin photocatalyst and a simple blue LED light source is reported.
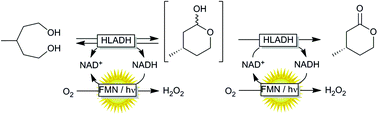
Green Chem., 2017,19, 376-379
https://doi.org/10.1039/C6GC02008A
Biocatalytic transamination with near-stoichiometric inexpensive amine donors mediated by bifunctional mono- and di-amine transaminases
Bifunctional transaminases enable a strategy for the production of chiral amines using small excesses of diamine donors.
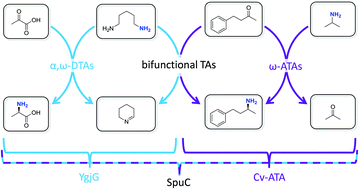
Green Chem., 2017,19, 361-366
https://doi.org/10.1039/C6GC02102F
Continuous flow biocatalysis: production and in-line purification of amines by immobilised transaminase from Halomonas elongata
The continuous flow synthesis of a series of amines was successfully achieved by exploiting the enhanced stability and broad substrate scope of an immobilised transaminase from Halomonas elongata (HEWT).
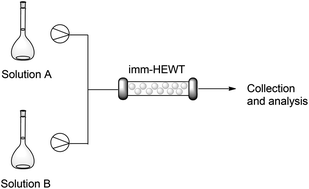
Green Chem., 2017,19, 372-375
https://doi.org/10.1039/C6GC01780K
From waste to value – direct utilization of limonene from orange peel in a biocatalytic cascade reaction towards chiral carvolactone
We investigated the valorisation of limonene containing waste product orange peel, and performed a biocatalytic cascade for the production of chiral carvolactone solely in water.
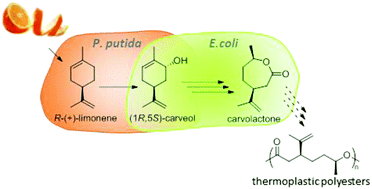
Green Chem., 2017,19, 367-371
https://doi.org/10.1039/C6GC01138A
Immobilization engineering – How to design advanced sol–gel systems for biocatalysis?
An immobilization engineering approach using bioinformatics and experimental design tools was applied to improve the sol–gel enzyme entrapment methodology.
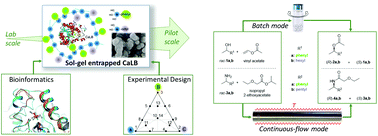
Green Chem., 2017,19, 3927-3937
https://doi.org/10.1039/C7GC00896A
Highly efficient and selective biocatalytic production of glucosamine from chitin
N-Acetyl glucosamine (GlcNAc) is one of the most abundant biomolecules on Earth and is cheaply available from chitin, a major component of crustaceans.
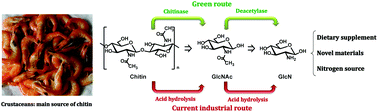
Green Chem., 2017,19, 527-535
https://doi.org/10.1039/C6GC02910H
Expanding the reaction space of aldolases using hydroxypyruvate as a nucleophilic substrate
Hydroxypyruvate was shown to be a nucleophile for class II pyruvate aldolases isolated from biodiversity, allowing unprecedented stereoselective cross-aldol reactions.
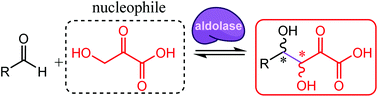
Green Chem., 2017,19, 519-526
https://doi.org/10.1039/C6GC02652D
Biocatalytic access to nonracemic γ-oxo esters via stereoselective reduction using ene-reductases
α,β-Unsaturated γ-keto esters were asymmetrically reduced by ene-reductases to yield versatile chiral intermediates in up to 99% ee.
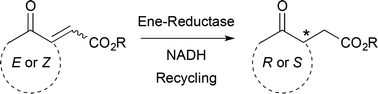
Green Chem., 2017,19, 511-518
https://doi.org/10.1039/C6GC02493A
Engineering a thermostable transketolase for arylated substrates
Transketolase variants were engineered to utilize arylalkanals and benzaldehyde as substrates with up to 28-fold rate acceleration for C–C bond formation with good yields (50–73%) and virtually complete (3S)-stereoselectivity (>99% ee).
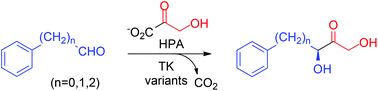
Green Chem., 2017,19, 481-489
https://doi.org/10.1039/C6GC02017H
Biocatalytic stereoinversion of D-para-bromophenylalanine in a one-pot three-enzyme reaction
D-Amino acid transaminase plus mutant phenylalanine dehydrogenase offer an effective one-pot system for 100% conversion of DL-amino acids to the L-form.
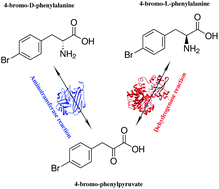
Green Chem., 2017,19, 503-510
https://doi.org/10.1039/C6GC01922F
Stereoselective amination of racemic sec-alcohols through sequential application of laccases and transaminases
A one-pot/two-step chemoenzymatic sequential methodology has been developed for the selective amination of secondary alcohols by combining the laccase from Trametes versicolor/TEMPO catalytic system with the stereoselective action of transaminases.
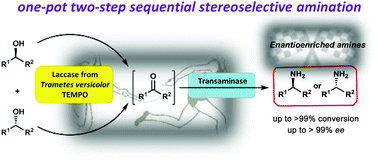
Green Chem., 2017,19, 474-480
https://doi.org/10.1039/C6GC01981A
Fully renewable polyesters via polycondensation catalyzed by Thermobifida cellulosilytica cutinase 1: an integrated approach
The present study addresses comprehensively the problem of producing polyesters through sustainable processes while using fully renewable raw materials and biocatalysts.
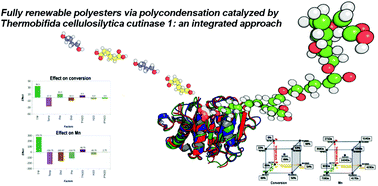
Green Chem., 2017,19, 490-502
https://doi.org/10.1039/C6GC02142E
Furfurylamines from biomass: transaminase catalysed upgrading of furfurals
A biocatalytic approach using transaminases has been used for the generation of a range of furfurylamines in good yields.
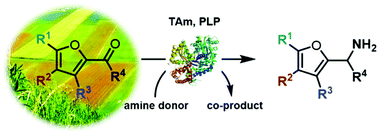
Green Chem., 2017,19, 397-404
https://doi.org/10.1039/C6GC02241C
New degradation compounds from lignocellulosic biomass pretreatment: routes for formation of potent oligophenolic enzyme inhibitors
In this study 26 new oligophenol cellulase inhibitors were discovered from wheat straw pretreatment liquors.

Green Chem., 2017,19, 464-473
https://doi.org/10.1039/C6GC01809B
New enzymatic methods for the synthesis of primary α-aminonitriles and unnatural α-amino acids by oxidative cyanation of primary amines with D-amino acid oxidase from porcine kidney
Oxidation of amino groups in amines or amino acids activates the sp3 Cα–H bond to form imines, making the alpha carbon atom a preferable target for nucleophilic reagents such as cyanide.
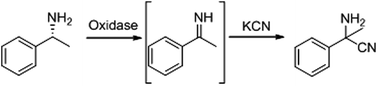
Green Chem., 2017,19, 418-424
https://doi.org/10.1039/C6GC02003H
Enzymatic halocyclization of allenic alcohols and carboxylates: a biocatalytic entry to functionalized O-heterocycles
Chloroperoxidase (C. fumago) catalyzes the aerobic halogenative 5-endo-trig cyclization of allenes in an aqueous emulsion system yielding functionalized O-heterocyclic building blocks.
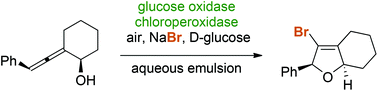
Green Chem., 2017,19, 447-452
https://doi.org/10.1039/C6GC01926A
One-pot, two-step cascade synthesis of naturally rare L-erythro (3S,4S) ketoses by coupling a thermostable transaminase and transketolase
Naturally rare L-erythro (3S,4S)-ketoses were prepared at high temperatures through a simultaneous two-step enzymatic cascade synthesis with excellent stereoselectivity.
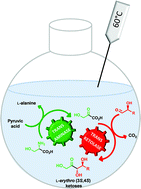
Green Chem., 2017,19, 425-435
https://doi.org/10.1039/C6GC02015A
Amine dehydrogenases: efficient biocatalysts for the reductive amination of carbonyl compounds
Optimised dual-enzyme (AmDH–FDH) reductive amination of a broad range of carbonyl compounds affords enantiopure amines with a conversion of up to 99% using ammonia as an amine donor and formate as a reducing reagent.
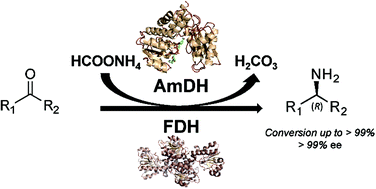
Green Chem., 2017,19, 453-463
https://doi.org/10.1039/C6GC01987K
Design of recombinant whole-cell catalysts for double reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and C
C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in enals and application in the synthesis of Guerbet alcohols as industrial bulk chemicals for lubricants
O bonds in enals and application in the synthesis of Guerbet alcohols as industrial bulk chemicals for lubricants
Whole-cell catalysts overexpressing two enzymes for a double reduction cascade in which aliphatic α-branched α,β-unsaturated aldehydes are converted into Guerbet alcohols as a highly demanded class of lubricants were constructed and applied in such biotransformations.
![Graphical abstract: Design of recombinant whole-cell catalysts for double reduction of C [[double bond, length as m-dash]] C and C [[double bond, length as m-dash]] O bonds in enals and application in the synthesis of Guerbet alcohols as industrial bulk chemicals for lubricants](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C6GC01668E&imageInfo.ImageIdentifier.Year=2017)
Green Chem., 2017,19, 405-410
https://doi.org/10.1039/C6GC01668E
Enantioselective chemoenzymatic synthesis of a key segment of neuronal nitric oxide synthase inhibitors and several related 3-aminopyridinylmethyl-4-hydroxypyrrolidines
High enantioselective lipase catalyzed resolution of several cis and trans 3-aminopyridinylmethyl-4-hydroxypyrrolidines has been described. Some of these pyrrolidines are precursors in the synthesis of selective nNOS inhibitors.
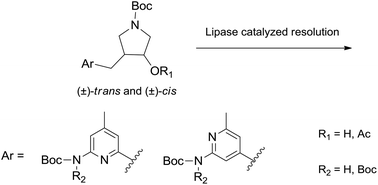
Green Chem., 2017,19, 436-446
https://doi.org/10.1039/C6GC01965J
Highly selective biocatalytic synthesis of monoacylglycerides in sponge-like ionic liquids
Monoacylglycerides are biocatalytically synthesized by direct esterification in sponge-like ionic liquids with high selectivity (e.g. up to 100% monolaurin in [C12mim][BF4]).
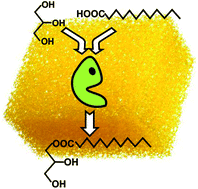
Green Chem., 2017,19, 390-396
https://doi.org/10.1039/C6GC01969B
Preparation of optically active cycloalkenes bearing all-carbon quaternary stereogenic centres via lipase–oxovanadium combo-catalysed dynamic kinetic resolution
A novel asymmetric synthesis of the title compounds was developed via the chemoenzymatic connection of three prochiral and achiral components.
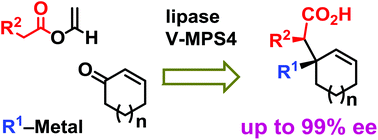
Green Chem., 2017,19, 411-417
https://doi.org/10.1039/C6GC01995A
About this collection
Guest-edited by Ulf Hanefeld (Technische Universiteit Delft) and Toshiyuki Itoh (Tottori University), this themed issue showcases recent research involving the use of enzyme catalysis for the production of bulk or fine chemicals, including that focussed on the development of efficient and clean processes using enzymes.