Highly selective biocatalytic synthesis of monoacylglycerides in sponge-like ionic liquids†
Abstract
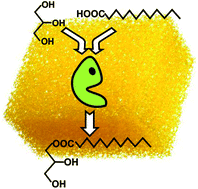
The biocatalytic synthesis of monoacylglycerides (MAGs) was carried out by the direct esterification of fatty acids (i.e. capric, lauric, myristic, palmitic and oleic acids, respectively) with glycerol in different ionic liquids (ILs) based on cations with long alkyl side-chains (e.g. 1-hexadecyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide [C16mim][NTf2], 1-dodecyl-3-methylimidazolium tetrafluoroborate [C12mim][BF4], etc.). Although all ILs have been shown as suitable reaction media for Novozym 435-catalyzed esterification of glycerol with free fatty acids, a high selectivity of MAGs was only observed in the [C12mim][BF4] case (e.g. up to 100% selectivity and 100% yield for monolaurin). Furthermore, as these ILs are temperature switchable ionic liquid/solid phases that behave as sponge-like systems, a straightforward protocol for IL-free MAG recovery, based on iterative centrifugations at controlled temperature, has been developed.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...