Enzymatic halocyclization of allenic alcohols and carboxylates: a biocatalytic entry to functionalized O-heterocycles†
Abstract
Chloroperoxidase from Caldariomyces fumago catalyzes the aerobic oxidative halocyclization of allenic alcohols and carboxylates yielding functionalized furan and pyran heterocycles as valuable synthetic scaffolds. Thanks to an oxidase-initiated redox cascade, simple halide salts – in combination with air and glucose – act as stoichiometric reagents for the in situ generation of reactive halonium species. Under the mild reaction conditions in an aqueous emulsion medium, the stereochemical integrity of diastereo- and enantioenriched allenes remains uncompromised and chiral dihydrofurans can be obtained via 5-endo-trig cyclizations with perfect axis-to-centre chirality transfer.
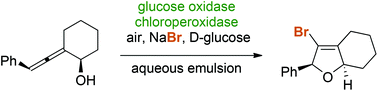
- This article is part of the themed collection: Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...