New enzymatic methods for the synthesis of primary α-aminonitriles and unnatural α-amino acids by oxidative cyanation of primary amines with d-amino acid oxidase from porcine kidney†
Abstract
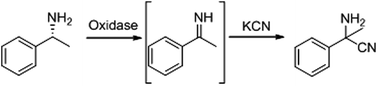
Oxidation of amino groups in amines or amino acids activates the sp3 Cα–H bond to form imines, making the alpha carbon atom a preferable target for nucleophilic reagents such as cyanide. Therefore, we focused on the oxidase reaction for the production of primary α-aminonitriles via imines. D-Amino acid oxidase from porcine kidney (pkDAO) and L-amino acid oxidase from Crotalus atrox catalyzed the synthesis of 2-amino-2-cyano-3-phenylpropanoic acid from phenylalanine and potassium cyanide (KCN). Mutant pkDAO (Y228L/R283G) catalyzed the synthesis of racemic-2-methyl-2-phenylglycinonitrile from (R)-α-methylbenzylamine and KCN. Based on these results, we developed a new cascade reaction for the synthesis of unnatural α-amino acids from primary amines using mutant pkDAO and nitrilase AY487533. This is the first report of the enzymatic synthesis of primary α-aminonitriles and unnatural α-amino acids. These methods will contribute widely to the synthesis of primary α-aminonitriles and unnatural α-amino acids in aqueous systems.
- This article is part of the themed collections: 2017 Green Chemistry Hot Articles and Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...