Abstract
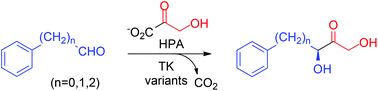
Aromatic components are difficult substrates for enzymes catalyzing stereoselective carboligation reactions. We have engineered transketolase from Geobacillus stearothermophilus by directed evolution to utilize arylalkanals and benzaldehyde as the electrophilic substrate in highly stereoselective C–C bond forming conversions. Enzyme variants were discovered with rate accelerations up to 28-fold that convert 2-phenylethanal, 3-phenylpropanal, phenyloxyethanal, benzyloxyethanal, and (N-Cbz)-3-aminopropanal with formation of the corresponding aryl-substituted 1,3-dihydroxyketones in good yields (60–72%) and virtually complete (3S)-stereoselectivity (>99% ee). Novel double-site variants were also found for the conversion of benzaldehyde.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis

 Please wait while we load your content...
Please wait while we load your content...