Amine dehydrogenases: efficient biocatalysts for the reductive amination of carbonyl compounds†
Abstract
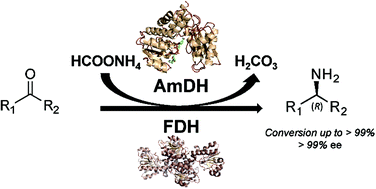
Amines constitute the major targets for the production of a plethora of chemical compounds that have applications in the pharmaceutical, agrochemical and bulk chemical industries. However, the asymmetric synthesis of α-chiral amines with elevated catalytic efficiency and atom economy is still a very challenging synthetic problem. Here, we investigated the biocatalytic reductive amination of carbonyl compounds employing a rising class of enzymes for amine synthesis: amine dehydrogenases (AmDHs). The three AmDHs from this study – operating in tandem with a formate dehydrogenase from Candida boidinii (Cb-FDH) for the recycling of the nicotinamide coenzyme – performed the efficient amination of a range of diverse aromatic and aliphatic ketones and aldehydes with up to quantitative conversion and elevated turnover numbers (TONs). Moreover, the reductive amination of prochiral ketones proceeded with perfect stereoselectivity, always affording the (R)-configured amines with more than 99% enantiomeric excess. The most suitable amine dehydrogenase, the optimised catalyst loading and the required reaction time were determined for each substrate. The biocatalytic reductive amination with this dual-enzyme system (AmDH–Cb-FDH) possesses elevated atom efficiency as it utilizes the ammonium formate buffer as the source of both nitrogen and reducing equivalents. Inorganic carbonate is the sole by-product.
- This article is part of the themed collection: Enzyme catalysis in organic synthesis


 Please wait while we load your content...
Please wait while we load your content...