Themed collection Natural Products

Broad-spectrum antitumor properties of Withaferin A: a proteomic perspective
A review discussing the broad-spectrum antitumor properties of the natural steroid Withaferin A based on the binding with its true cancer protein targets: a defined stress dependent and stress independent mode of action.
RSC Med. Chem., 2020,11, 30-50
https://doi.org/10.1039/C9MD00296K
Recent insights into natural product inhibitors of matrix metalloproteinases
Members of the matrix metalloproteinase (MMP) family have biological functions that are central to human health and disease, and MMP inhibitors have been investigated for the treatment of cardiovascular disease, cancer and neurodegenerative disorders.
Med. Chem. Commun., 2019,10, 2024-2037
https://doi.org/10.1039/C9MD00165D
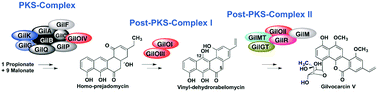
Post-PKS enzyme complexes
This review highlights the protein–protein interactions between type II post-PKS tailoring enzymes with an emphasis on gilvocarcin and mithramycin.
Med. Chem. Commun., 2019,10, 1855-1866
https://doi.org/10.1039/C9MD00066F
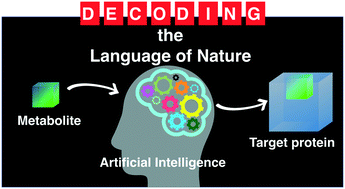
Is it time for artificial intelligence to predict the function of natural products based on 2D-structure
One of chemistry's grand challenges is to find a function for every known metabolite. We explore the opportunity for artificial intelligence to provide rationale interrogation of metabolites to predict their function.
Med. Chem. Commun., 2019,10, 1667-1677
https://doi.org/10.1039/C9MD00128J
3-Ketoacyl-ACP synthase (KAS) III homologues and their roles in natural product biosynthesis
KAS III-like enzymes play a significant role in natural product biosynthesis through C–C, C–O, and/or C–N bond formation.

Med. Chem. Commun., 2019,10, 1517-1530
https://doi.org/10.1039/C9MD00162J
Diversification of polyketide structures via synthase engineering
We present examples of polyketide structure diversification along with a perspective on the present and future of polyketide synthetic biology.
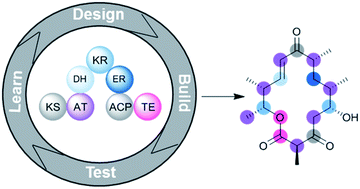
Med. Chem. Commun., 2019,10, 1256-1272
https://doi.org/10.1039/C9MD00141G
Using Drosophila as a platform for drug discovery from natural products in Parkinson's disease
The review provides an overview of discovery of new drug leads from natural extracts using Drosophila as a screening platform to evaluate the therapeutic potential of phytochemicals against Parkinson's disease.
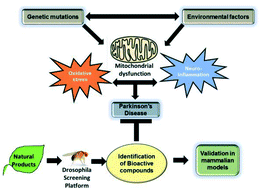
Med. Chem. Commun., 2019,10, 867-879
https://doi.org/10.1039/C9MD00099B
Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018)
This review covers advances made in genome mining SMs produced by Aspergillus nidulans, Aspergillus fumigatus, Aspergillus niger, and Aspergillus terreus in the past six years (2012–2018). Genetic identification and molecular characterization of SM biosynthetic gene clusters, along with proposed biosynthetic pathways, is discussed in depth.
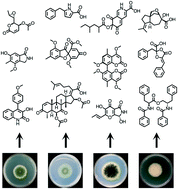
Med. Chem. Commun., 2019,10, 840-866
https://doi.org/10.1039/C9MD00054B
Leveraging synthetic biology for producing bioactive polyketides and non-ribosomal peptides in bacterial heterologous hosts
A review discussing the role of heterologous expression in the discovery and engineered production of bioactive polyketides and non-ribosomal peptides from bacteria.
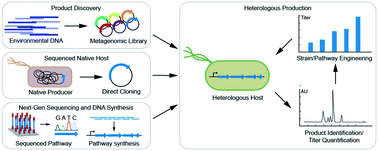
Med. Chem. Commun., 2019,10, 668-681
https://doi.org/10.1039/C9MD00055K
A practical synthesis of amino limonin/deoxylimonin derivatives as effective mitigators against inflammation and nociception
A practical synthetic route, consisting of 5 steps, has been developed and applied successfully for converting limonin/deoxylimonin into the corresponding amino derivatives I-5a–I-5e and II-5a–II-5e.
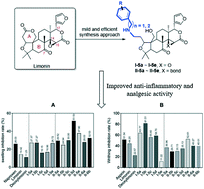
RSC Med. Chem., 2020,11, 843-847
https://doi.org/10.1039/D0MD00117A
FgaPT2, a biocatalytic tool for alkyl-diversification of indole natural products
Demonstration of FgaPT2 catalyzed alkyl-diversification of indole containing natural products.
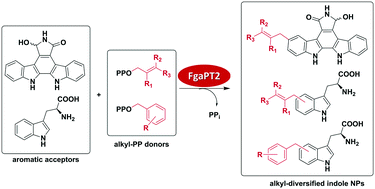
Med. Chem. Commun., 2019,10, 1465-1475
https://doi.org/10.1039/C9MD00177H
The winding road of the uvaretin class of natural products: from total synthesis to bioactive agent discovery
Herein, we disclose the development of a synthetic route to gain access to the uvaretin class of chalcone natural products.
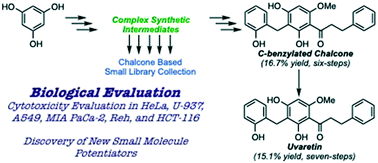
Med. Chem. Commun., 2019,10, 1420-1431
https://doi.org/10.1039/C9MD00052F
Synthesis of N-acyl amide natural products using a versatile adenylating biocatalyst
TamA is the enzyme that controls the acyl chain length of the tambjamine natural products. Here we show that the catalytic ANL domain of TamA can be used to prepare a range of N-acyl amides.
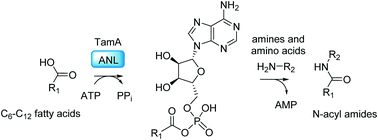
Med. Chem. Commun., 2019,10, 1192-1196
https://doi.org/10.1039/C9MD00063A
Nodulisporic acid E biosynthesis: in vivo characterisation of NodD1, an indole-diterpene prenyltransferase that acts on an emindole SB derived indole-diterpene scaffold
Prenylation of aromatic compounds is a key tailoring reaction in biosynthesis of bioactive indole-diterpenes.

Med. Chem. Commun., 2019,10, 1160-1164
https://doi.org/10.1039/C9MD00143C
Synthesis and direct comparison of the anticancer activities of phomopsolides D and E and two 7-oxa-/7-aza-analogues
The synthesis and biological evaluation of two phomopsolide natural products (D and E) and two analogues is presented.
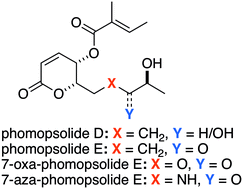
Med. Chem. Commun., 2019,10, 1205-1211
https://doi.org/10.1039/C9MD00121B
Fungal natural alkaloid schizocommunin activates the aryl hydrocarbon receptor pathway
Activation of AhR by schizocommunin is linked to increased expression of xenobiotic metabolizing enzymes associated with immune and allergic responses.
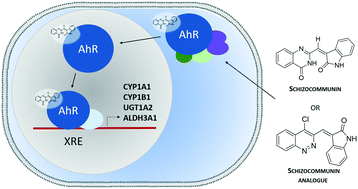
Med. Chem. Commun., 2019,10, 985-990
https://doi.org/10.1039/C9MD00138G
Construction of a hybrid gene cluster to reveal coupled ring formation–hydroxylation in the biosynthesis of HSAF and analogues from Lysobacter enzymogenes
HSAF and analogues are polycyclic tetramate macrolactams (PoTeMs) isolated from Lysobacter enzymogenes.
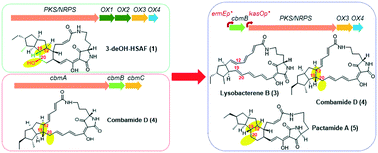
Med. Chem. Commun., 2019,10, 907-912
https://doi.org/10.1039/C9MD00154A
Stereoselective semi-synthesis of the neuroprotective natural product, serofendic acid
Our synthesis of neuroprotectant serofendic acid from biosourced ent-atis-16-en-19-oic acid represents the best route to date.
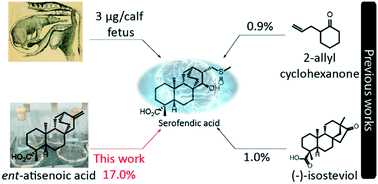
Med. Chem. Commun., 2019,10, 951-960
https://doi.org/10.1039/C9MD00145J
Synthesis, conformational preferences, and biological activity of conformational analogues of the microtubule-stabilizing agents, (−)-zampanolide and (−)-dactylolide
Zampanolide and dactylolide are microtubule-stabilizing polyketides possessing potent cytotoxicity towards a variety of cancer cell lines.
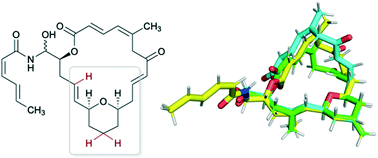
Med. Chem. Commun., 2019,10, 800-805
https://doi.org/10.1039/C9MD00164F
How mithramycin stereochemistry dictates its structure and DNA binding function
The crystal structures of mithramycin and its analogue reveal how the chemistry of mithramycin shapes it for DNA binding.
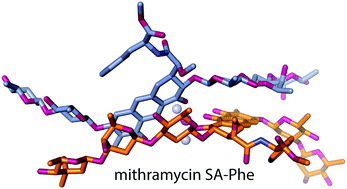
Med. Chem. Commun., 2019,10, 735-741
https://doi.org/10.1039/C9MD00100J
Volatiles from the ascomycete Daldinia cf. childiae (Hypoxylaceae), originating from China
The volatiles from an isolate of the fungus Daldinia cf. childiae, obtained from a specimen collected in China, were collected by use of a closed-loop stripping apparatus and analysed by GC-MS.
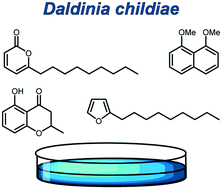
Med. Chem. Commun., 2019,10, 726-734
https://doi.org/10.1039/C9MD00083F
Synthesis of endolides A and B: naturally occurring N-methylated cyclic tetrapeptides
The first syntheses of the bioactive cyclic tetrapeptide natural products, endolides A and B, were accomplished using a solution-phase macrocyclisation reaction; the stereoselectivity of which was found to be reagent-controlled.
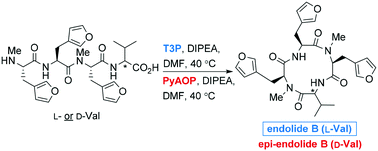
Med. Chem. Commun., 2019,10, 693-698
https://doi.org/10.1039/C9MD00050J
Origin and bioactivities of thiosulfinated FK228
During a large laboratory-scale purification of FK228 from the fermentation broth of Burkholderia thailandensis MSMB43, a small amount of thiosulfinated FK228 (TS-FK228) was unexpectedly purified only after the broth was mixed with silica gel.
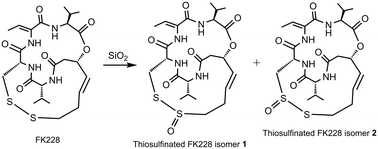
Med. Chem. Commun., 2019,10, 538-542
https://doi.org/10.1039/C9MD00060G
Isolation and characterization of tambjamine MYP1, a macrocyclic tambjamine analogue from marine bacterium Pseudoalteromonas citrea
Identification of a macrocyclic tambjamine natural product, tambjamine MYP1, from a marine bacterium that may enhance bioactivity by restraining bond rotation.
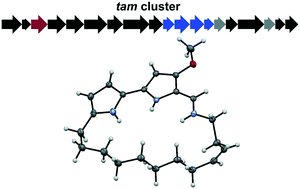
Med. Chem. Commun., 2019,10, 478-483
https://doi.org/10.1039/C9MD00061E