Synthesis of endolides A and B: naturally occurring N-methylated cyclic tetrapeptides†
Abstract
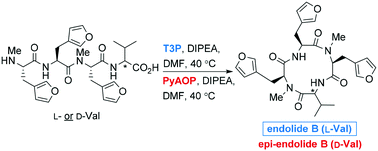
Endolides A and B are naturally occurring, N-methylated, cyclic tetrapeptides possessing an unusual 3-(3-furyl)alanine amino acid and outstanding biological profiles. 1-Propanephosphonic anhydride (T3P) was used to mediate a solution-phase cyclisation reaction of the linear tetrapeptides, thus achieving the first syntheses of both endolides A and B. The stereoselectivity of the tetrapeptide cyclisation reactions was found to be reagent-controlled, and was independent of the C-terminal configuration of the linear peptide starting materials.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...