A practical synthesis of amino limonin/deoxylimonin derivatives as effective mitigators against inflammation and nociception†
Abstract
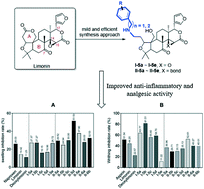
A practical synthetic route, consisting of 5 steps, has been developed and applied successfully for converting limonin/deoxylimonin into the corresponding amino derivatives I-5a–I-5e and II-5a–II-5e. Deoxylimonin analogs II-5a and II-5b bearing an open A ring structure underwent ring closure reaction by employing the Mitsunobu procedure to afford II-6a and II-6b. All of the 12 newly synthesized compounds were subjected to preliminary screening for evaluating their inflammation and nociception inhibition efficacy. The most promising compounds, I-5b and II-5d, were selected for further investigation by a carrageenan-induced mouse paw edema model, both of which displayed a dose–response dependent manner and demonstrated superior anti inflammation capacity to that of indomethacin in the first 2 hours.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...