Synthesis of N-acyl amide natural products using a versatile adenylating biocatalyst†
Abstract
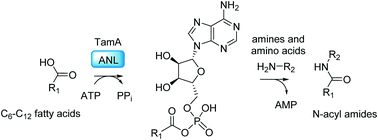
Natural products are secondary metabolites produced by many different organisms such as bacteria, fungi and plants. These biologically active molecules have been widely exploited for clinical application. Here we investigate TamA, a key enzyme from the biosynthetic pathway of tambjamine YP1, an acylated bipyrrole that is produced by the marine microorganism Pseudoalteromonas tunicata. TamA is a didomain enzyme composed of a catalytic adenylation (ANL) and an acyl carrier protein (ACP) domain that together control the fatty acid chain length of the YP1. Here we show that the TamA ANL domain alone can be used to generate a range of acyl adenylates that can be captured by a number of amines thus leading to the production of a series of fatty N-acyl amides. We exploit this biocatalytic promiscuity to produce the recently discovered class of N-acyl histidine amide natural products from Legionella pneumophila.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...