Synthesis, conformational preferences, and biological activity of conformational analogues of the microtubule-stabilizing agents, (−)-zampanolide and (−)-dactylolide†
Abstract
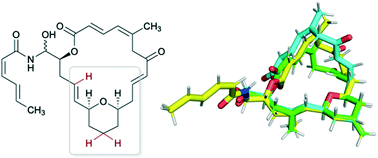
Zampanolide and dactylolide are microtubule-stabilizing polyketides possessing potent cytotoxicity towards a variety of cancer cell lines. Using our understanding of the conformational preferences of the macrolide core in both natural products, we hypothesized that analogues lacking the C17-methyl group would maintain the necessary conformation for bioactivity while reducing the number of synthetic manipulations necessary for their synthesis. Analogues 3, 4 and 5 were prepared via total synthesis, and their conformational preferences were determined through computational and high-field NMR studies. While no observable activities were present in dactylolide analogues 3 and 4, zampanolide analogue 5 exhibited sub-micromolar cytotoxicity. Herein, we describe these efforts towards understanding the structure- and conformation-activity relationships of dactylolide and zampanolide.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...