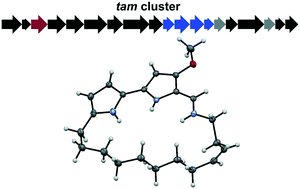
Isolation and characterization of tambjamine MYP1, a macrocyclic tambjamine analogue from marine bacterium Pseudoalteromonas citrea†
Abstract
Tambjamines are natural products that consist of a conserved bipyrrole core functionalized with different imines giving rise to many derivatives. The core structure of tambjamines allows ion coordination through the nitrogen atoms, which is a key aspect in many of their observed antimicrobial, anticancer, and antimalarial bioactivities. Minor variances in the compound structure have a considerable impact on the potency of these activities, so identifying new analogues is valuable for maximizing tambjamine biological potential. In this work, we describe the isolation and structure elucidation of the first naturally occurring macrocyclized tambjamine, tambjamine MYP1, from the marine microbe Pseudoalteromonas citrea. We also compare the apparent pKa of cyclic and linear tambjamine analogues and discuss how structural strain may effect the compound's ion coordination abilities.
- This article is part of the themed collection: Natural Products


 Please wait while we load your content...
Please wait while we load your content...