Themed collection 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis

Light opens a new window for N-heterocyclic carbene catalysis
This minireview summarized the recent advances on the photoinduced, NHC-catalyzed organic reactions according to the function of visible light.

Chem. Sci., 2020,11, 10605-10613
https://doi.org/10.1039/D0SC03595E
Recent advances in N-heterocyclic carbene-based radical catalysis
This minireview examines the history and state-of-the-art of the N-heterocyclic carbene-based radical catalysis.
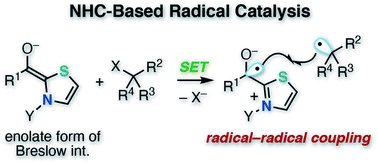
Chem. Sci., 2020,11, 5630-5636
https://doi.org/10.1039/D0SC01538E
Tale of the Breslow intermediate, a central player in N-heterocyclic carbene organocatalysis: then and now
Molecular insights on the formation, detection, and even isolation of the Breslow intermediate, which is the most important species in N-heterocyclic carbene (NHC) catalysis, as obtained from experimental and computational studies, are presented.
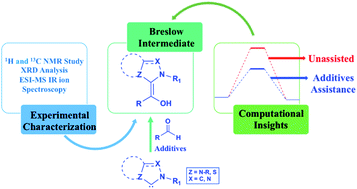
Chem. Sci., 2021,12, 7973-7992
https://doi.org/10.1039/D1SC01910D
Organocatalytic asymmetric formal oxidative coupling for the construction of all-aryl quaternary stereocenters
A one-pot oxidation of racemic triarylmethanes to form para-quinone methides followed by enantioselective construction of all-aryl quaternary stereocenters has been developed.
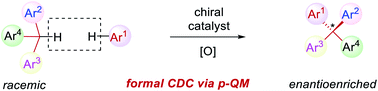
Chem. Sci., 2021,12, 11793-11798
https://doi.org/10.1039/D1SC03324G
Stereodivergent entry to β-branched β-trifluoromethyl α-amino acid derivatives by sequential catalytic asymmetric reactions
A one-pot approach to β-branched β-trifluoromethyl α-amino acids, grounded on the reduction – ring opening of Erlenmeyer–Plöchl azlactones, and complementary to conventional catalytic asymmetric hydrogenation, is presented.

Chem. Sci., 2021,12, 10233-10241
https://doi.org/10.1039/D1SC01442K
Reaction-based machine learning representations for predicting the enantioselectivity of organocatalysts
A machine learning model for enantioselectivity prediction using reaction-based molecular representations.
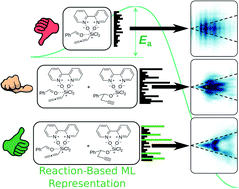
Chem. Sci., 2021,12, 6879-6889
https://doi.org/10.1039/D1SC00482D
Construction of chiral α-tert-amine scaffolds via amine-catalyzed asymmetric Mannich reactions of alkyl-substituted ketimines
Stereoselective Mannich reactions of aldehydes with ketimines provide chiral β-amino aldehydes that bear an α-tert-amine moiety.
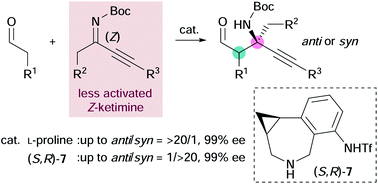
Chem. Sci., 2021,12, 1445-1450
https://doi.org/10.1039/D0SC05269H
Evidence for an enolate mechanism in the asymmetric Michael reaction of α,β-unsaturated aldehydes and ketones via a hybrid system of two secondary amine catalysts
The key nucleophile was found to be neither an enamine nor an enol, but an enolate in the direct Michael reaction of α,β-unsaturated aldehydes and non-activated ketones catalyzed by two amine catalysts namely diphenylprolinol silyl ether and pyrrolidine.

Chem. Sci., 2020,11, 11293-11297
https://doi.org/10.1039/D0SC03359F
Origin of rate enhancement and asynchronicity in iminium catalyzed Diels–Alder reactions
Quantum chemical activation strain analyses revealed that iminium catalysts accelerate Diels–Alder reactions by reducing the Pauli repulsion between reactants.
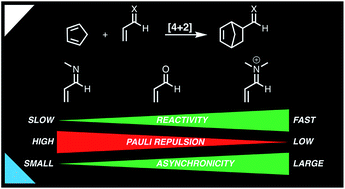
Chem. Sci., 2020,11, 8105-8112
https://doi.org/10.1039/D0SC02901G
Allylic C(sp3)–H alkylation via synergistic organo- and photoredox catalyzed radical addition to imines
A new catalytic method for the direct alkylation of allylic C(sp3)–H bonds from unactivated alkenes via synergistic organo- and photoredox catalysis is described.
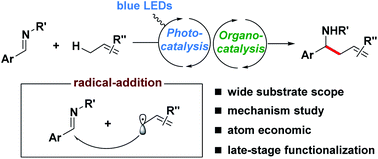
Chem. Sci., 2020,11, 4954-4959
https://doi.org/10.1039/D0SC00819B
Enantioselective aerobic oxidative cross-dehydrogenative coupling of glycine derivatives with ketones and aldehydes via cooperative photoredox catalysis and organocatalysis
A visible-light-induced enantioselective aerobic oxidative cross-dehydrogenative coupling between glycine derivatives and simple ketones or aldehydes is achieved.
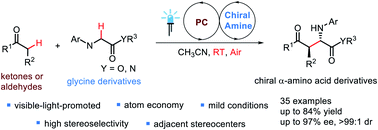
Chem. Sci., 2020,11, 4741-4746
https://doi.org/10.1039/D0SC00683A
What is the role of acid–acid interactions in asymmetric phosphoric acid organocatalysis? A detailed mechanistic study using interlocked and non-interlocked catalysts
Supramolecular acid–acid interactions lead to competing monomeric and dimeric pathways in phosphoric acid catalysis – so that stereoselectivities depend on catalyst concentration.
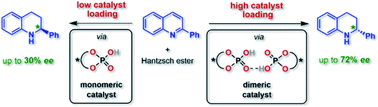
Chem. Sci., 2020,11, 4381-4390
https://doi.org/10.1039/D0SC01026J
Enhancing the selectivity of prolinamide organocatalysts using the mechanical bond in [2]rotaxanes
The mechanical bonding and the cofactor assembly in interlocked prolinamide-based organocatalysts upgrade enamine-type transformations by increasing their yields and enantio- and chemo-selectivities.
![Graphical abstract: Enhancing the selectivity of prolinamide organocatalysts using the mechanical bond in [2]rotaxanes](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=D0SC00444H&imageInfo.ImageIdentifier.Year=2020)
Chem. Sci., 2020,11, 3629-3635
https://doi.org/10.1039/D0SC00444H
Isothiourea-catalysed enantioselective Michael addition of N-heterocyclic pronucleophiles to α,β-unsaturated aryl esters
The isothiourea-catalysed enantioselective Michael addition of 3-aryloxindole and 4-substituted-dihydropyrazol-3-one pronucleophiles to α,β-unsaturated p-nitrophenyl esters is reported.
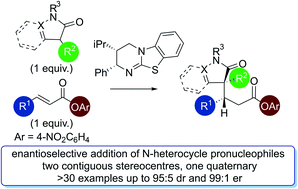
Chem. Sci., 2020,11, 241-247
https://doi.org/10.1039/C9SC04303A
Multigram-scale flow synthesis of the chiral key intermediate of (−)-paroxetine enabled by solvent-free heterogeneous organocatalysis
The continuous flow synthesis of the chiral key intermediate of (−)-paroxetine is demonstrated via a solvent-free organocatalytic conjugate addition followed by a telescoped reductive amination–lactamization–amide/ester reduction sequence.
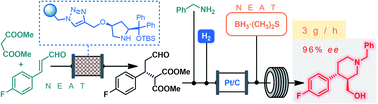
Chem. Sci., 2019,10, 11141-11146
https://doi.org/10.1039/C9SC04752B
Enantioselective total synthesis of the unnatural enantiomer of quinine
A practical enantioselective total synthesis of the unnatural (+)-quinine and (−)-9-epi-quinine enantiomers, which are important organocatalysts, is reported.
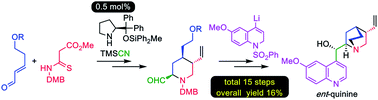
Chem. Sci., 2019,10, 9433-9437
https://doi.org/10.1039/C9SC03879E
Proline bulky substituents consecutively act as steric hindrances and directing groups in a Michael/Conia-ene cascade reaction under synergistic catalysis
In this study, we report a highly stereoselective and versatile synthesis of spiro pyrazolones, promising motifs that are being employed as pharmacophores.
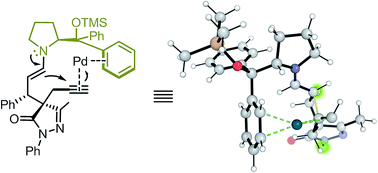
Chem. Sci., 2019,10, 4107-4115
https://doi.org/10.1039/C8SC05258A
Oxidative organocatalysed enantioselective coupling of indoles with aldehydes that forms quaternary carbon stereocentres
The first organocatalysed, metal-free cross-nucleophile coupling of indoles with α-branched aldehydes forming acyclic stereoselective quaternary carbon centres is presented.
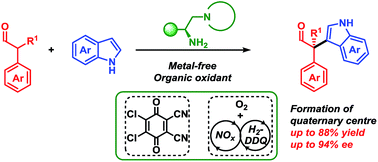
Chem. Sci., 2019,10, 3586-3591
https://doi.org/10.1039/C9SC00196D
Site-selective C–C modification of proteins at neutral pH using organocatalyst-mediated cross aldol ligations
An organocatalyst-mediated protein aldol ligation (OPAL) affords C–C linked bioconjugates at neutral pH.
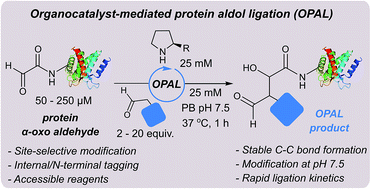
Chem. Sci., 2018,9, 5585-5593
https://doi.org/10.1039/C8SC01617H
Enantioselective direct Mannich-type reactions of 2-benzylpyridine N-oxides catalyzed by chiral bis(guanidino)iminophosphorane organosuperbase
2-Benzylpyridine N-oxides possessing less acidic α-protons were utilized as pronucleophiles for the first time in enantioselective addition reactions under Brønsted base catalysis.
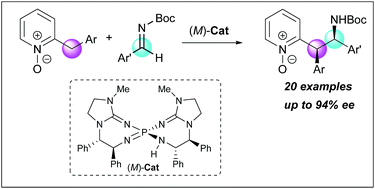
Chem. Sci., 2018,9, 4348-4351
https://doi.org/10.1039/C8SC00808F
Total synthesis of diazonamide A
A total synthesis of diazonamide A is reported, featuring a highly stereoselective organocatalytic construction of the challenging C(10) quaternary center.
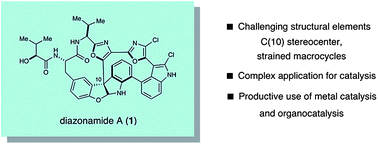
Chem. Sci., 2011,2, 308-311
https://doi.org/10.1039/C0SC00577K
About this collection
The Nobel Prize in Chemistry 2021 was jointly awarded to Benjamin List and David W.C. MacMillan for the development of asymmetric organocatalysis. As the founding Editor-in-Chief for Chemical Science, David played a visionary role for scientific publishing, with the journal still being a home for some of the very best organic chemistry and catalysis research published. Our Associate Editor Paolo Melchiorre has selected outstanding articles in Chemical Science highlighting some of the fantastic work currently ongoing in the area of asymmetric organocatalysis.