Multigram-scale flow synthesis of the chiral key intermediate of (−)-paroxetine enabled by solvent-free heterogeneous organocatalysis†
Abstract
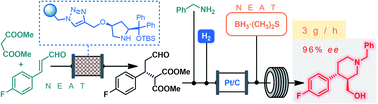
The catalytic enantioselective synthesis of the chiral key intermediate of the antidepressant (−)-paroxetine is demonstrated as a continuous flow process on multi-gram scale. The critical step is a solvent-free organocatalytic conjugate addition followed by a telescoped reductive amination–lactamization–amide/ester reduction sequence. Due to the efficient heterogeneous catalysts and the solvent-free or highly concentrated conditions applied, the flow method offers key advances in terms of productivity and sustainability compared to earlier batch approaches.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...