Organocatalytic asymmetric formal oxidative coupling for the construction of all-aryl quaternary stereocenters†
Abstract
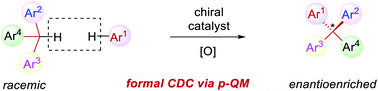
A new catalytic asymmetric formal cross dehydrogenative coupling process for the construction of all-aryl quaternary stereocenters is disclosed, which provides access to rarely explored chiral tetraarylmethanes with excellent enantioselectivity. The suitable oxidation conditions and the hydrogen-bond-based organocatalysis have enabled efficient intermolecular C–C bond formation in an overwhelmingly crowded environment under mild conditions. para-Quinone methides bearing an ortho-directing group serve as the key intermediate. The precise loading of DDQ is critical to the high enantioselectivity. The chiral products have also been demonstrated as promising antiviral agents.
- This article is part of the themed collection: 2021 Nobel Prize in Chemistry – Asymmetric Organocatalysis


 Please wait while we load your content...
Please wait while we load your content...