Themed collection Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

π-Acidic alkene ligand effects in Pd-catalysed cross-coupling processes: exploiting the interaction of dibenzylidene acetone (dba) and related ligands with Pd(0) and Pd(II)
Alkene ligands exert interesting properties on Pd(0) and Pd(II) species, which can be exploited in catalytic processes.
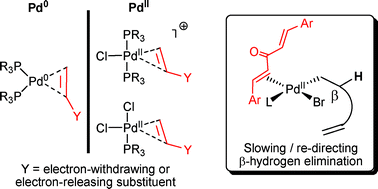
Org. Biomol. Chem., 2008,6, 3645-3656
https://doi.org/10.1039/B811772A
The Heck–Mizoroki cross-coupling reaction: a mechanistic perspective
Recent advances in the study of the mechanism of the Heck–Mizoroki reaction are presented and discussed.
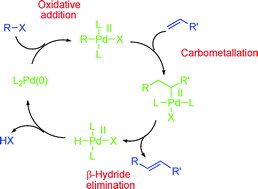
Org. Biomol. Chem., 2007,5, 31-44
https://doi.org/10.1039/B611547K
Cross-coupling reactions of nucleoside triphosphates followed by polymerase incorporation. Construction and applications of base-functionalized nucleic acids
Novel construction of base-functionalized nucleic acids consisting in cross-coupling reactions of nucleoside triphosphates followed by polymerase incorporation is presented within the context of a minireview on enzymatic synthesis of base-modified DNA and RNA.
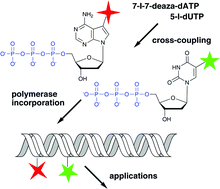
Org. Biomol. Chem., 2008,6, 2233-2241
https://doi.org/10.1039/B803664K
Platinum -catalyzed cross-dehydrogenative coupling reaction in the absence of oxidant
A third strategy for cross-dehydrogenative coupling reaction has been reported via platinum catalysis in the absence of oxidant.
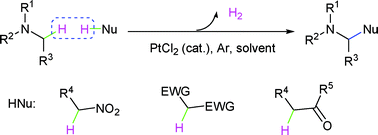
Org. Biomol. Chem., 2010,8, 4077-4079
https://doi.org/10.1039/C0OB00261E
Nickel-catalyzed cross-coupling of diarylamines with haloarenes
The cross-coupling reaction of diarylamines with aryl bromides/iodides can be effected by the Ni(II)–(σ-aryl) complex/PPh3/NaH system, and a preliminary investigation was conducted into the mechanism of this reaction.
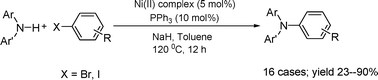
Org. Biomol. Chem., 2009,7, 3922-3925
https://doi.org/10.1039/B911286C
Fluoride-free cross coupling using vinyldisiloxanes
Vinyldisiloxanes equilibrate with the corresponding silanolates under basic conditions and subsequently undergo palladium catalysed cross coupling with aryl/heteroaryl iodides and bromides.
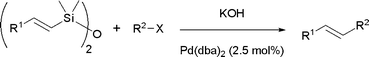
Org. Biomol. Chem., 2009,7, 1068-1072
https://doi.org/10.1039/B900852G
Palladium-catalyzed decarboxylative cross-coupling reaction of cinnamic acid with aryl iodide
A highly effective decarboxylative cross-coupling reaction of cinnamic acid with aryl iodide catalyzed by the combination of palladium chloride and CyJohnPhos is described.
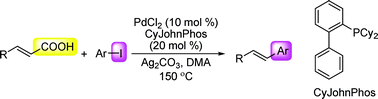
Org. Biomol. Chem., 2009,7, 863-865
https://doi.org/10.1039/B821870F
Suzuki–Miyaura cross-coupling reaction catalyzed by Pd/MgLa mixed oxide
A new, reusable Pd/MgLa mixed oxide catalyst has been applied successfully to the Suzuki–Miyaura carbon–carbon cross-coupling reaction.
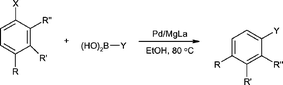
Org. Biomol. Chem., 2005,3, 4307-4309
https://doi.org/10.1039/B512767J
Facile synthesis of multisubstituted buta-1,3-dienes via Suzuki–Miyaura and Kumada cross-coupling strategy of 2,4-diiodo-buta-1-enes with arylboronic acids and Grignard reagents
One-pot Suzuki–Miyaura-type and Kumada-type cross-coupling reactions of 2,4-diiodo-buta-1-enes with arylboronic acids and alkyl/aryl magnesium bromides were carried out in the presence of accessibly simple catalysts under mild conditions.
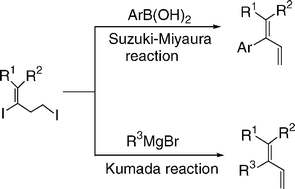
Org. Biomol. Chem., 2005,3, 1828-1831
https://doi.org/10.1039/B504071J
Tandem one pot asymmetric conjugate addition–vinyl triflate formation–cross coupling methodology
Optically active vinyl triflates are obtained and employed in a series of one pot metal-catalyzed tandem asymmetric transformations.
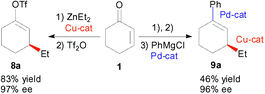
Org. Biomol. Chem., 2005,3, 729-731
https://doi.org/10.1039/B418028C
Exploiting the palladium[0]-catalysed Ullmann cross-coupling reaction in natural products chemistry: application to a total synthesis of the alkaloid (±)-aspidospermidine
Azide 14, available through the title cross-coupling process, has been converted, via the ring-fused aziridine 15, into the alkaloid aspidospermidine.
![Graphical abstract: Exploiting the palladium[0]-catalysed Ullmann cross-coupling reaction in natural products chemistry: application to a total synthesis of the alkaloid (±)-aspidospermidine](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B415977B&imageInfo.ImageIdentifier.Year=2005)
Org. Biomol. Chem., 2005,3, 213-215
https://doi.org/10.1039/B415977B
Cross-coupling reaction of alcohols for carbon–carbon bond formation using pincer-type NHC/palladium catalysts
A cross-coupling reaction of different alcohols was achieved using a pincer-type NHC/PdBr complex as the catalyst precursor, and the reaction, under either Ar or H2 gas, displayed a broad substrate scope with respect to both primary and secondary alcohol components, with high product alcohol selectivity.
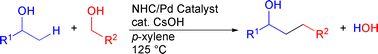
Org. Biomol. Chem., 2010,8, 896-900
https://doi.org/10.1039/B914618K
[2.2]Paracyclophane -based monophosphine ligand for palladium-catalyzed cross-coupling reactions of aryl chlorides
A bulky, electron-rich monophosphine has been prepared, showing excellent performance in the Pd-catalyzed Buchwald–Hartwig amination and Suzuki–Miyaura coupling reaction of aryl chlorides, including deactivated ones.
![Graphical abstract: [2.2]Paracyclophane-based monophosphine ligand for palladium-catalyzed cross-coupling reactions of aryl chlorides](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B906139H&imageInfo.ImageIdentifier.Year=2009)
Org. Biomol. Chem., 2009,7, 3236-3242
https://doi.org/10.1039/B906139H
New asymmetric synthesis of protein farnesyltransferase inhibitors via palladium -catalyzed cross-coupling reactions of 2-iodo-imidazoles
The Suzuki methodology was found to be the most effective palladium catalyzed cross-coupling reaction to synthesize in few steps enantiomerically pure 2-propylsuccinyl imidazole derivatives from chiral alkenyl boronate esters.
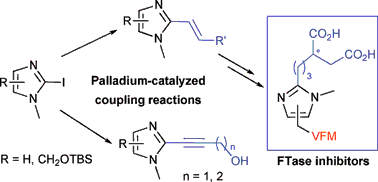
Org. Biomol. Chem., 2009,7, 2214-2222
https://doi.org/10.1039/B902601K
N-heterocycle
carbene (NHC)-ligated cyclopalladated N,N-dimethylbenzylamine : a highly active, practical and versatile catalyst for the Heck–Mizoroki reaction
An easily prepared, air- and moisture-stable, well-defined NHC-palladacycle mediates the single and double Heck–Mizoroki reaction of a number of functionalized aryl bromides, iodides and triflates with acrylic esters, amides and styrene derivatives in good to excellent yields.
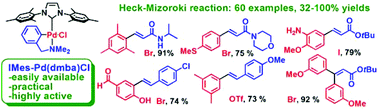
Org. Biomol. Chem., 2009,7, 2110-2119
https://doi.org/10.1039/B821892G
Trifluoromethyl -substituted pyridyl- and pyrazolylboronic acids and esters : synthesis and Suzuki–Miyaura cross-coupling reactions
Highly-functionalised pyridine and pyrazole derivatives incorporating trifluoromethyl substituents have been synthesised by Suzuki–Miyaura cross-coupling protocols.
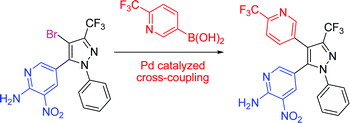
Org. Biomol. Chem., 2009,7, 2155-2161
https://doi.org/10.1039/B901024F
Alcohol cross-coupling reactions catalyzed by Ru and Ir terpyridine complexes
Primary alcohols can be coupled with secondary benzylic alcohols by an air-stable catalytic system involving terpyridine ruthenium or iridium complexes.
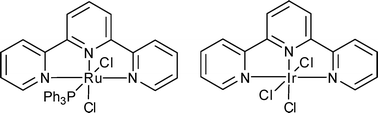
Org. Biomol. Chem., 2008,6, 4442-4445
https://doi.org/10.1039/B815547J
Phosphinates as new electrophilic partners for cross-coupling reactions
Lactam derived enol phosphinates are novel viable electrophiles in Suzuki–Miyaura and Stille cross-coupling reactions.
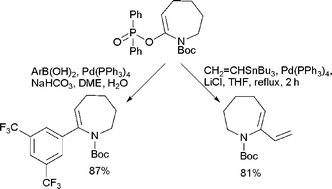
Org. Biomol. Chem., 2008,6, 4053-4058
https://doi.org/10.1039/B809577A
Palladium(0)-catalyzed direct cross-coupling reaction of allylic alcohols with aryl- and alkenylboronic acids
Allylic alcohols can be used directly for the palladium-catalyzed allylation of aryl- and alkenylboronic acids without the aid of a base.
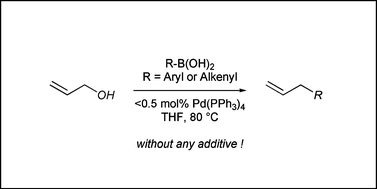
Org. Biomol. Chem., 2008,6, 3005-3013
https://doi.org/10.1039/B804991B
Synthesis of substituted 6-cyclopropylpurine bases and nucleosides by cross-coupling reactions or cyclopropanations
Synthesis of novel purine bases and nucleosides bearing unsubstituted or substituted cyclopropyl rings in position 6 is reported. 6-Cyclopropylpurine ribonucleoside exerted a significant cytostatic effect while all substituted derivatives were inactive.
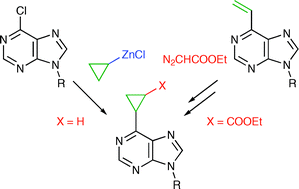
Org. Biomol. Chem., 2008,6, 2377-2387
https://doi.org/10.1039/B802833H
Synthesis of 2′-deoxyadenosine nucleosides bearing bipyridine-type ligands and their Ru-complexes in position 8 through cross-coupling reactions
Title 2′-deoxyadenosine derivatives bearing bipyridine, phenanthroline or terpyridine ligands and their RuII-complexes in position 8 were prepared by cross-couplings of 8-bromoadenosine with acetylene or boronate derivatives of oligopyridine ligands or Ru complexes.
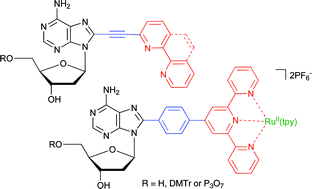
Org. Biomol. Chem., 2007,5, 2849-2857
https://doi.org/10.1039/B709245H
Efficient Heck reactions catalyzed by a highly recyclable palladium(II) complex of a pyridyl-functionalized imidazolium-based ionic liquid
A series of pyridyl-functionalized imidazolium-based ionic liquids with high thermal stability and wide liquid range were synthesized. Heck cross-coupling reactions catalyzed by a Pd(II)-containing ionic liquid complex in the corresponding ionic liquid shows high recyclability.
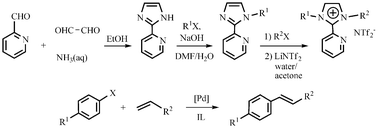
Org. Biomol. Chem., 2007,5, 671-678
https://doi.org/10.1039/B616529J
Insights into the mechanism of the site-selective sequential palladium -catalyzed cross-coupling reactions of dibromothiophenes/dibromothiazoles and arylboronic acids. Synthesis of PPARβ/δ agonists
A reactivity study, aided by NMR spectroscopy, leads to conclusions regarding aspects like positional selectivity and rate-limiting steps. Additionally, trisubstituted thiophene derivatives with PPARβ/δ agonist activity have been synthesized.
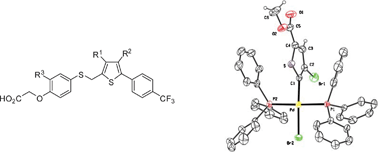
Org. Biomol. Chem., 2006,4, 4514-4525
https://doi.org/10.1039/B612235C
Cross-coupling reactions of unprotected halopurine bases, nucleosides , nucleotides and nucleoside triphosphates with 4-boronophenylalanine in water . Synthesis of (purin-8-yl)- and (purin-6-yl)phenylalanines
(Adenin-8-yl)phenylalanine base, nucleosides, nucleotides and nucleoside triphosphates, as well as (purin-6-yl)phenylalanine base and nucleosides were prepared by aqueous-phase Pd-catalysed cross-coupling reactions.
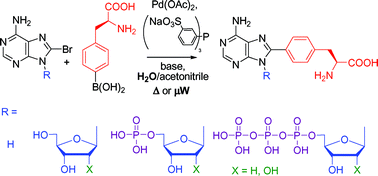
Org. Biomol. Chem., 2006,4, 2278-2284
https://doi.org/10.1039/B604010A
Copper(I) mediated cross-coupling of amino acid derived organozinc reagents with acid chlorides
A user-friendly protocol for the copper(I) mediated cross-coupling of acid chlorides with organozinc reagents derived from amino acids.
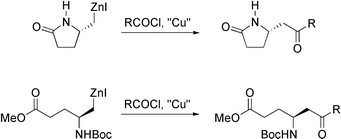
Org. Biomol. Chem., 2006,4, 1796-1805
https://doi.org/10.1039/B601996J
Synthesis and electronic properties of series of oligothiophene-[1,10]phenanthrolines
Synthesis of a variety of oligothiophene-substituted [1,10]phenanthrolines in good overall yields and purities by Pd-catalyzed Negishi cross-coupling reactions of iodinated phenanthrolines and zincated oligothiophenes.
![Graphical abstract: Synthesis and electronic properties of series of oligothiophene-[1,10]phenanthrolines](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B508572A&imageInfo.ImageIdentifier.Year=2005)
Org. Biomol. Chem., 2005,3, 4143-4152
https://doi.org/10.1039/B508572A
Dye -functionalized head-to-tail coupled oligo(3-hexylthiophenes)—perylene –oligothiophene dyads for photovoltaic applications
Novel donor–acceptor hybrid molecules, consisting of head-to-tail coupled oligo(3-hexylthiophene)s covalently linked to perylenemonoimide, were synthesized and structure–property relationships established.
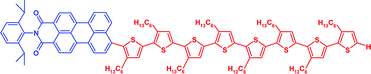
Org. Biomol. Chem., 2005,3, 985-995
https://doi.org/10.1039/B414817G
An exploration of Suzuki aryl cross coupling chemistry involving [2.2]paracyclophane derivatives
An examination into the scope and limitations of Suzuki aryl cross coupling chemistry using derivatives of [2.2]paracyclophane is described.
![Graphical abstract: An exploration of Suzuki aryl cross coupling chemistry involving [2.2]paracyclophane derivatives](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=B415764H&imageInfo.ImageIdentifier.Year=2005)
Org. Biomol. Chem., 2005,3, 515-519
https://doi.org/10.1039/B415764H
About this collection
To commemorate the 2010 Nobel Prize for Chemistry awarded to Heck, Negishi and Suzuki for their contributions to Pd-catalised cross coupling reactions in organic synthesis we have put together a collection of some of the Organic & Biomolecular Chemistry articles recently published on this groundbreaking subject.
Congratulations to these three pioneers of organic chemistry.