Insights into the mechanism of the site-selective sequential palladium-catalyzed cross-coupling reactions of dibromothiophenes/dibromothiazoles and arylboronic acids. Synthesis of PPARβ/δ agonists†
Abstract
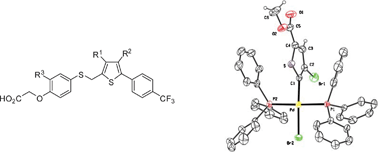
A reactivity study, aided by
- This article is part of the themed collection: Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

 Please wait while we load your content...
Please wait while we load your content...