Efficient Heck reactions catalyzed by a highly recyclable palladium(ii) complex of a pyridyl-functionalized imidazolium-based ionic liquid†
Abstract
The reactions of
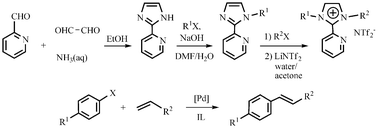
- This article is part of the themed collection: Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

 Please wait while we load your content...
Please wait while we load your content...