Dye-functionalized head-to-tail coupled oligo(3-hexylthiophenes)—perylene–oligothiophene dyads for photovoltaic applications†
Abstract
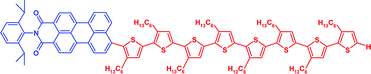
A series of novel donor–acceptor systems, consisting of head-to-tail coupled oligo(3-hexylthiophene)s covalently linked to perylenemonoimide, is described. These hybrid molecules, which differ by the length of the oligothiophene units from a monothiophene up to an octathiophene, were created via effective
- This article is part of the themed collection: Chemistry Nobel 2010 Web Collection: Cross-coupling reactions in organic chemistry

 Please wait while we load your content...
Please wait while we load your content...