Themed collection Non-traditional solvent effects in organic reactions

Non-traditional solvent effects in organic reactions
This special cross-journal collection of Organic and Biomolecular Chemistry (OBC) and Physical Chemistry Chemical Physics (PCCP) is dedicated to non-traditional solvent effects in organic reactions.

Phys. Chem. Chem. Phys., 2021,23, 26028-26029
https://doi.org/10.1039/D1CP90187G
Deep eutectic solvents as non-traditionally multifunctional media for the desulfurization process of fuel oil
This review summarizes the physicochemical properties of deep eutectic solvents (DESs) and their applications for the desulfurization processes of fuel oil. Moreover, current challenges and future opportunity are discussed.
Phys. Chem. Chem. Phys., 2021,23, 785-805
https://doi.org/10.1039/D0CP05153E
Advances in deep eutectic solvents and water: applications in metal- and biocatalyzed processes, in the synthesis of APIs, and other biologically active compounds
This review highlights recent advances in metal- and biocatalyzed transformations, in the synthesis of APIs and other biologically active compounds, when employing deep eutectic solvents and water as environmentally responsible solvents.
Org. Biomol. Chem., 2021,19, 2558-2577
https://doi.org/10.1039/D0OB02491K
Ionic liquids: “normal” solvents or nanostructured fluids?
This review provides an overview of the literature from 2010 to the present day, covering the effect of ionic liquids (ILs) on organic reactivity. Two major viewpoints emerge, based on linear solvation energy relationships or nanostructure of ILs.
Org. Biomol. Chem., 2021,19, 2076-2095
https://doi.org/10.1039/D0OB02214D
The role of intermolecular forces in ionic reactions: the solvent effect, ion-pairing, aggregates and structured environment
A general view of the medium effects on ionic reactions involves the solvent effect, ion pairing, formation of aggregates and structured environment.
Org. Biomol. Chem., 2021,19, 1900-1914
https://doi.org/10.1039/D0OB02413A
How the external solvent in biocompatible reverse micelles can improve the alkaline phosphatase behavior
Hydrolysis of 1-naphthyl phosphate by alkaline phosphatase in biocompatible reverse micelles.

Org. Biomol. Chem., 2021,19, 4969-4977
https://doi.org/10.1039/D0OB02371J
Formation of water-in-oil microemulsions within a hydrophobic deep eutectic solvent
The neoteric, non-toxic, and inexpensive hydrophobic deep eutectic solvents as potential alternative to toxic organic solvents as oil phase in water-in-oil microemulsions.
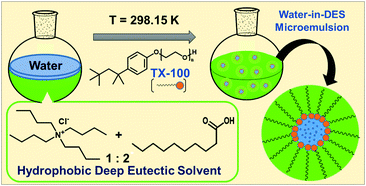
Phys. Chem. Chem. Phys., 2021,23, 10629-10635
https://doi.org/10.1039/D0CP06716D
Rapid relaxation NMR measurements to predict rate coefficients in ionic liquid mixtures. An examination of reaction outcome changes in a homologous series of ionic liquids
Rate coefficient and spin–spin relaxation time measurements are used to understand and predict solvent effects in ionic liquids.
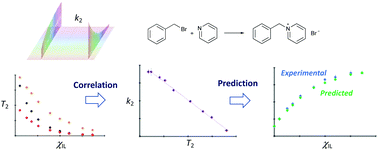
Phys. Chem. Chem. Phys., 2021,23, 9878-9888
https://doi.org/10.1039/D0CP06066F
Aerobic α-hydroxylation of 2-Me-1-tetralone in 1-alkyl-3-methylimidazolium ionic liquids
The aerobic α-hydroxylation of 2-Me-1-tetralone was investigated in imidazol-based ionic liquids (ILs), where the mesoscopic structure of ILs was found crucial for the reaction rate.

Phys. Chem. Chem. Phys., 2021,23, 5864-5869
https://doi.org/10.1039/D0CP06047J
Taking advantage of lithium monohalocarbenoid intrinsic α-elimination in 2-MeTHF: controlled epoxide ring-opening en route to halohydrins
Lithium monohalocarbenoids are useful synthons for conducting C1-homologations, although their high tendency to undergo degradative α-elimination, leading to a lithium halide and a free carbene, is the main drawback for expanding their use in synthesis.

Org. Biomol. Chem., 2021,19, 2038-2043
https://doi.org/10.1039/D0OB02407D
Copper-catalyzed Goldberg-type C–N coupling in deep eutectic solvents (DESs) and water under aerobic conditions
A scalable CuI-catalyzed Goldberg C–N coupling between aryl iodides and amides is reported either in DESs or in water.

Org. Biomol. Chem., 2021,19, 1773-1779
https://doi.org/10.1039/D0OB02501A
Nucleophilic degradation of diazinon in thermoreversible polymer–polymer aqueous biphasic systems
Polymer–polymer aqueous two-phase systems involve thermoreversible reaction–separation processes in the nucleophilic degradation of diazinon and further separation of the reaction products.
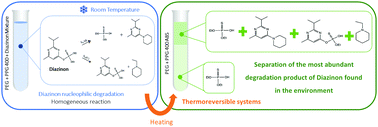
Phys. Chem. Chem. Phys., 2021,23, 4133-4140
https://doi.org/10.1039/D0CP06086K
Insights on the catalytic behaviour of sulfonic acid-functionalized ionic liquids (ILs) in transesterification reactions – voltammetric characterization of sulfonic task-specific ILs with bisulfate anions
Cyclic voltammetry measurements on imidazolic ionic liquids with sulfonic acid groups and bisulfate counterions revealed that they work as efficient reservoirs of sulfuric acid and are able to dose it on demand to uphold an efficient acid catalysis.
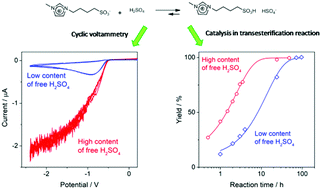
Phys. Chem. Chem. Phys., 2021,23, 2731-2741
https://doi.org/10.1039/D0CP05674J
Machine learning approaches to understand and predict rate constants for organic processes in mixtures containing ionic liquids
Machine learning models were developed for an organic reaction in ionic liquids and validated on a selection of ionic liquids.
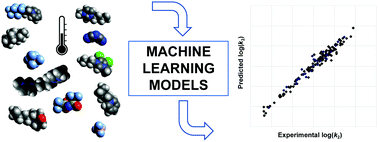
Phys. Chem. Chem. Phys., 2021,23, 2742-2752
https://doi.org/10.1039/D0CP04227G
On the role of water in the hydrogen bond network in DESs: an ab initio molecular dynamics and quantum mechanical study on the urea–betaine system
We herein report an ab initio molecular dynamics study on a natural DES composed of urea and betaine in a 3 : 2 ratio, as a test case for evaluating the water effect.
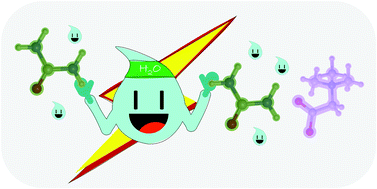
Phys. Chem. Chem. Phys., 2021,23, 1994-2004
https://doi.org/10.1039/D0CP06078J
Hydrogen bond donor functionalized poly(ionic liquid)s for efficient synergistic conversion of CO2 to cyclic carbonates
A series of heterogeneous catalysts, combined hydrogen bond donors and ionic liquids, are reported for efficient CO2 cycloaddition with epoxides.
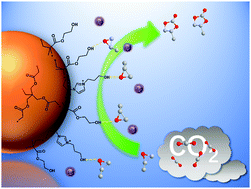
Phys. Chem. Chem. Phys., 2021,23, 2005-2014
https://doi.org/10.1039/D0CP06041K
Mechanistic insights into carbon dioxide utilization by superoxide ion generated electrochemically in ionic liquid electrolyte
Understanding the reaction mechanism that controls the one-electron electrochemical reduction of oxygen is essential for sustainable use of the superoxide ion (O2˙−) for CO2 conversion.
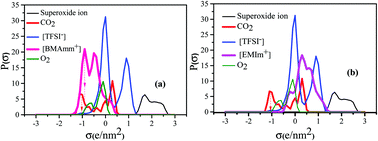
Phys. Chem. Chem. Phys., 2021,23, 1114-1126
https://doi.org/10.1039/D0CP04903D
Deep eutectic solvent as solvent and catalyst: one-pot synthesis of 1,3-dinitropropanes via tandem Henry reaction/Michael addition
1,3-Dinitropropane derivatives were obtained in a simple one-pot procedure from nitromethane and aromatic aldehydes by combining MW irradiation and the use of DES as both catalyst and solvent.
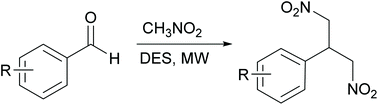
Org. Biomol. Chem., 2020,18, 8395-8401
https://doi.org/10.1039/D0OB01516D
Controlling the outcome of SN2 reactions in ionic liquids: from rational data set design to predictive linear regression models
An iterative, combined experimental and computational approach towards predicting reaction rate constants in ionic liquids is presented.
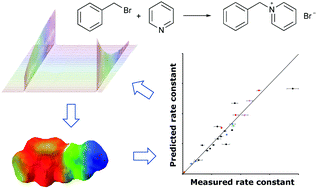
Phys. Chem. Chem. Phys., 2020,22, 23009-23018
https://doi.org/10.1039/D0CP04224B
Microwave-assisted nucleophilic degradation of organophosphorus pesticides in propylene carbonate
Degradation of organophosphorus pesticides was achieved by using microwaves, an ionic liquid and propylene carbonate.

Org. Biomol. Chem., 2020,18, 7868-7875
https://doi.org/10.1039/D0OB01620A
The effect of bisimidazolium-based ionic liquids on a bimolecular substitution process. Are two head(group)s better than one?
Bisimidazolium-based ionic liquids generally result in greater rate constants than the corresponding monocationic salts. The effect depends on the length of the bridging chain; likely due to a change in the mode of interactions in solution.
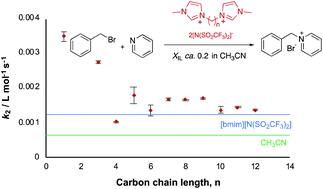
Org. Biomol. Chem., 2020,18, 7388-7395
https://doi.org/10.1039/D0OB01500H
The multifaceted effects of DMSO and high hydrostatic pressure on the kinetic constants of hydrolysis reactions catalyzed by α-chymotrypsin
The cosolvent DMSO and high pressure have antagonistic effects on the kinetic constants of α-chymotrypsin-catalyzed hydrolysis reactions.
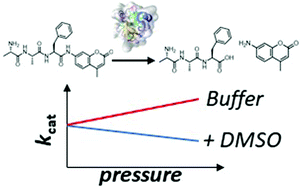
Phys. Chem. Chem. Phys., 2020,22, 16325-16333
https://doi.org/10.1039/D0CP03062G
Controlling the reactions of 1-bromogalactose acetate in methanol using ionic liquids as co-solvents
Using an ionic liquid in the solvent mixture for the reaction of a galactose substrate leads to changes in both the rate constant and the products as the solvent composition changes.
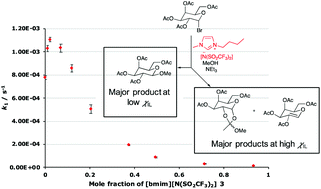
Org. Biomol. Chem., 2020,18, 5442-5452
https://doi.org/10.1039/D0OB01198C
About this collection
This special cross-journal collection of Organic and Biomolecular Chemistry (OBC) and Physical Chemistry Chemical Physics (PCCP) is dedicated to non-traditional solvent effects in organic reactions. The scope of this collection covers all aspects of solvent effects on organic reactivity - from the physical underpinnings through to synthetic utility in the areas of preparative, physical organic, and computational chemistry. A broad range of non-traditional solvents are covered including supercritical fluids, superheated solvents, eutectic mixtures, and ionic liquids.
Guest Edited by: Jason Harper (University of New South Wales), Barbara Kirchner (Universität Bonn), Paulina Pavez G. (Pontificia Universidad Católica de Chile) and Tom Welton (Imperial College London).