Themed collection Trends in Organoboron Chemistry

Organoboron synthesis via ring opening coupling reactions
This review covers new trends towards the selective synthesis of organoboron compounds where boron reagents and cyclic substrates participate in the generation of carbanions, in the presence of stoichiometric amounts of main-group metals or catalytic amounts of transition metal complexes, via ring opening coupling transformations.

Org. Biomol. Chem., 2019,17, 6317-6325
https://doi.org/10.1039/C9OB00989B
Recent advances in the borylative transformation of carbonyl and carboxyl compounds
The recent advances in the borylative transformation of carbonyl and carboxyl compounds are summarized.
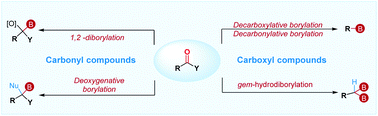
Org. Biomol. Chem., 2019,17, 6099-6113
https://doi.org/10.1039/C9OB01029G
Pyridine-catalyzed desulfonative borylation of benzyl sulfones
Herein, we report the transition-metal free, pyridine-catalyzed desulfonative borylation of benzyl sulfones with bis(pinacolato)diboron (B2pin2).
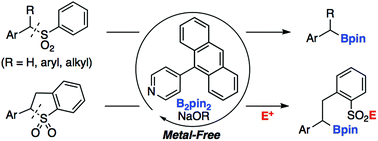
Org. Biomol. Chem., 2019,17, 7300-7303
https://doi.org/10.1039/C9OB01099H
Amine-tethered phenylboronic acid-enabling ring-opening strategy for carbon chain elongation from double aldol cyclic hemiacetals
A boron reagent for the ring-opening reaction of double aldol cyclic hemiacetals to generate their aldehyde forms is developed.
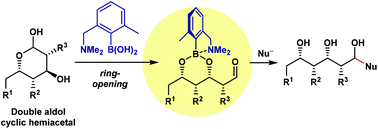
Org. Biomol. Chem., 2019,17, 6562-6565
https://doi.org/10.1039/C9OB01263J
(Hetero)arylboration of alkynes: a strategy for the synthesis of α,α-bis(hetero)arylketones
A method for (hetero)arylboration of alkynes is presented.
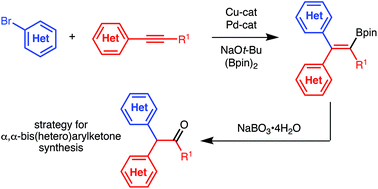
Org. Biomol. Chem., 2019,17, 5913-5915
https://doi.org/10.1039/C9OB00961B
Biocompatible conjugation of Tris base to 2-acetyl and 2-formyl phenylboronic acid
Tris, a commonly used buffering agent, readily reacts with 2-acetyl/formyl phenylboronic acid in complex biological media, yielding conjugates with kinetic stability similar to oximes.
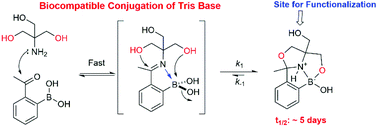
Org. Biomol. Chem., 2019,17, 5908-5912
https://doi.org/10.1039/C9OB00726A
Copper-mediated anomeric O-arylation with organoboron reagents
Copper-mediated couplings of boroxines with glycosyl hemiacetals generate O-aryl glycosides via Csp2–O bond formation.
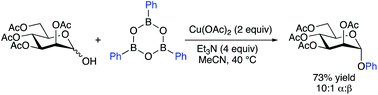
Org. Biomol. Chem., 2019,17, 5671-5674
https://doi.org/10.1039/C9OB01022J
Synthesis of 2-alkyl-2-boryl-substituted-tetrahydrofurans via copper(I)-catalysed borylative cyclization of aliphatic ketones
A new method was developed for synthesizing 2-alkyl-2-boryl-tetrahydrofuran derivatives from aliphatic ketones using a copper(I)/N-heterocyclic carbene complex catalyst.
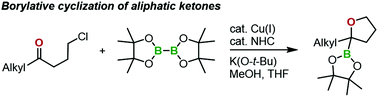
Org. Biomol. Chem., 2019,17, 5680-5683
https://doi.org/10.1039/C9OB00962K
Borylative cyclisation of diynes using BCl3 and borocations
Products from the borylative cyclisation of diynes using BCl3 is dependent on substituent effects, however, some control of product outcome is achieved using borocations or BCl3 in the presence of [BCl4]−.
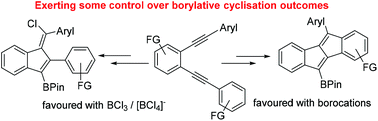
Org. Biomol. Chem., 2019,17, 5520-5525
https://doi.org/10.1039/C9OB00991D
Planarized B,N-phenylated dibenzoazaborine with a carbazole substructure: electronic impact of the structural constraint
Structural constraint in B,N-phenylated dibenzoazaborine alters the π-conjugation mode and furnishes intense deep-blue emission and intriguing electrochemical properties.
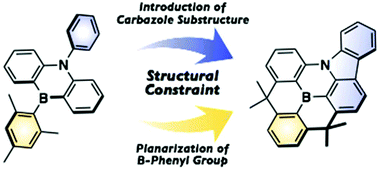
Org. Biomol. Chem., 2019,17, 5500-5504
https://doi.org/10.1039/C9OB00934E
NHC-copper-thiophene-2-carboxylate complex for the hydroboration of terminal alkynes
An air-stable N-heterocyclic carbene–copper thiophene-2-carboxylate (CuTC) complex has been prepared for the stereoselective hydroboration of terminal alkynes using pinacolborane (HBpin) or 1,8-naphthalenediaminatoborane (HBdan).
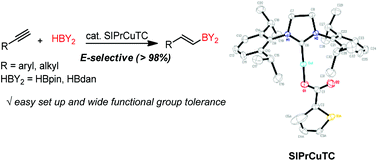
Org. Biomol. Chem., 2019,17, 5249-5252
https://doi.org/10.1039/C9OB00839J
9-Borabicyclo[3.3.l]nonane-induced Friedel–Crafts benzylation of arenes with benzyl fluorides
9-Borabicyclo[3.3.l]nonane dimer (9-BBN) mediates the Friedel–Crafts benzylation of arenes with benzyl fluorides, affording a series of 1,1-diarylmethanes under mild conditions.
![Graphical abstract: 9-Borabicyclo[3.3.l]nonane-induced Friedel–Crafts benzylation of arenes with benzyl fluorides](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9OB00912D&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 5258-5261
https://doi.org/10.1039/C9OB00912D
Thiolation of NHC-boranes: influence of the substitution at boron
B-Substituted NHC-boranes react with aryldisulfides to generate mono-thiolated compounds selectively under both ionic or radical conditions.
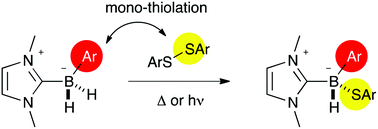
Org. Biomol. Chem., 2019,17, 4234-4237
https://doi.org/10.1039/C9OB00578A
Exploring the strength of a hydrogen bond as a function of steric environment using 1,2-azaborine ligands and engineered T4 lysozyme receptors
The relationship between the steric demand of the ligand and hydrogen bonding strength in the context of ligand–protein binding is revealed using engineered T4 lysozymes as the model biomacromolecules and 1,2-azaborines as ligands.
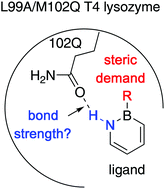
Org. Biomol. Chem., 2019,17, 7002-7006
https://doi.org/10.1039/C9OB01008D
Copper-catalysed borylation of aryl chlorides
The first example of a Cu-catalysed borylation of a wide range of aryl chlorides with different electronic and steric properties is mediated by a readily prepared NHC-stabilised Cu catalyst and KOtBu. The aryl chlorides are converted into their corresponding arylboronic esters using B2pin2 or B2neop2 as the boron reagent.
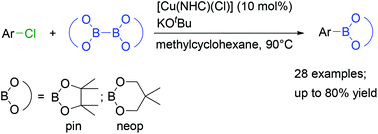
Org. Biomol. Chem., 2019,17, 6601-6606
https://doi.org/10.1039/C9OB01244C
Catalytic direct amidations in tert-butyl acetate using B(OCH2CF3)3
B(OCH2CF3)3-catalysed direct amidations of challenging substrates (polar heteroycles, poorly nucleophilic anilines) work well in tBuOAc under Dean–Stark conditions.
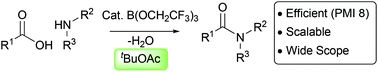
Org. Biomol. Chem., 2019,17, 6465-6469
https://doi.org/10.1039/C9OB01012B
Push–pull isomers of indolizino[6,5,4,3-def]phenanthridine decorated with a triarylboron moiety
Push–pull regioisomers decorated with a triarylboron unit and an electron-withdrawing aryl ring based on an unsymmetrical indolizino[6,5,4,3-def]phenanthridine backbone have been found to display distinct temperature “turn-on” fluorescence.
![Graphical abstract: Push–pull isomers of indolizino[6,5,4,3-def]phenanthridine decorated with a triarylboron moiety](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9OB00923J&imageInfo.ImageIdentifier.Year=2019)
Org. Biomol. Chem., 2019,17, 6470-6477
https://doi.org/10.1039/C9OB00923J
Bis-aminocyclopropenylidene carbene borane catalyzed imine hydrogenation
Borenium-catalyzed hydrogenations of an imine made from a benzylamine are enabled by a steric interplay between cyclohexylborane and a BAC carbene: both are necessary for efficient reactivity.
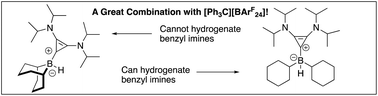
Org. Biomol. Chem., 2019,17, 6158-6164
https://doi.org/10.1039/C9OB01053J
Two-component boronic acid catalysis for increased reactivity in challenging Friedel–Crafts alkylations with deactivated benzylic alcohols
The combination of a boronic acid catalyst with perfluoropinacol as a co-catalyst improves the scope of Friedel–Crafts benzylations of arenes with electronically deactivated primary and secondary benzylic alcohols.
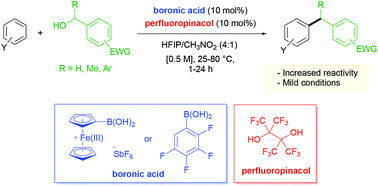
Org. Biomol. Chem., 2019,17, 6007-6014
https://doi.org/10.1039/C9OB01043B
Metal-free synthesis of gem-silylboronate esters and their Pd(0)-catalyzed cross-coupling with aryliodides
An efficient method for the synthesis of gem-silylboronate esters based on transition-metal-free reaction of arylboronic acids and trimethylsilyldiazomethane is developed, and Suzuki–Miyaura cross-coupling of gem-silylboronate esters with aryliodides is described.
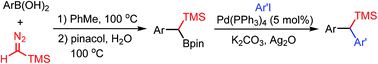
Org. Biomol. Chem., 2019,17, 5714-5724
https://doi.org/10.1039/C9OB01006H
Ni vs. Pd in Suzuki–Miyaura sp2–sp2 cross-coupling: a head-to-head study in a comparable precatalyst/ligand system
A head-to-head study of comparable pre-catalysts in Suzuki–Miyaura cross-coupling.

Org. Biomol. Chem., 2019,17, 5055-5059
https://doi.org/10.1039/C9OB00561G
Aryl–aryl coupling in a polycyclic aromatic hydrocarbon with embedded tetracoordinate boron centre
An oxaboraphenanthrene is generated from a 9,10-dihydro-9,10-diboraanthracene through boron extrusion/C–C coupling upon addition of mesityl lithium and chromatographic workup.
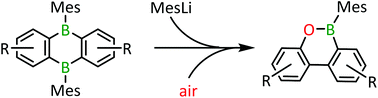
Org. Biomol. Chem., 2019,17, 5060-5065
https://doi.org/10.1039/C9OB00618D