Bis-aminocyclopropenylidene carbene borane catalyzed imine hydrogenation†
Abstract
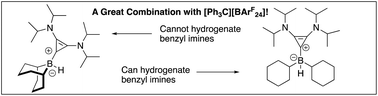
Certain borenium cations supported by carbenes can function as hydrogenation catalysts for imines. While many carbenes have been explored, variation of the other groups on boron has been less common. We have investigated several carbene–borane adducts in an attempt to understand the ability of a bis-amino cyclopropenylidene (BAC) carbene dicyclohexylborane adduct to hydrogenate relatively sterically unhindered benzyl imines. As an additional variant, a BAC carbene adduct of diphenylborane was prepared. A convenient preparation of diphenylboron fluoride via a potassium fluoroborinate salt was employed in this chemistry. Reaction of diphenylboron fluoride with a BAC carbene afforded a modest yield of a carbene–fluoroborane adduct. Reaction between the fluoroborinate salt and a lithium tetrafluoroborate adduct of the carbene provided the adduct in much improved yield and cleanliness, and the product was structurally characterized. The fluoroborate could be converted to a boron hydride through fluoride–hydride exchange with dimethylchlorosilane. The boron hydride adduct was also structurally characterized. Unlike the BAC carbene dicyclohexylborane adduct, the BAC carbene diphenylborane adduct showed essentially no activity in hydrogenation of imines or enamines.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...