Copper-mediated anomeric O-arylation with organoboron reagents†
Abstract
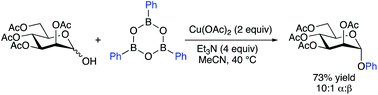
Copper-mediated couplings of arylboroxines with glycosyl hemiacetals furnish O-aryl glycosides via Csp2–O bond formation. The method enables the anomeric O-arylation of protected pyranose and furanose derivatives, and is tolerant of functionalized arylboroxine partners. Whereas mixtures of anomers are formed from glucopyranose, galactopyranose and arabinofuranose hemiacetals, the α-anomer is generated selectively from mannopyranose and mannofuranose-derived substrates.
- This article is part of the themed collections: Chemical Biology in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...