Pyridine-catalyzed desulfonative borylation of benzyl sulfones†
Abstract
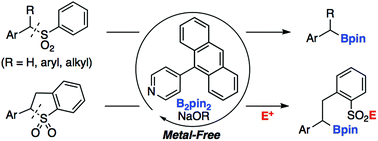
Herein, we report the transition-metal free, pyridine-catalyzed desulfonative borylation of benzyl sulfones with bis(pinacolato)diboron (B2pin2). A variety of benzhydryl- and benzyl boronic esters could be synthesized from readily prepared sulfone derivatives. The borylation of cyclic sulfones accompanied by ring opening also proceeded to afford the corresponding sulfonate, which could be converted into functionalized sulfones and sulfonamides.
- This article is part of the themed collections: Catalysis & biocatalysis in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...