Biocompatible conjugation of Tris base to 2-acetyl and 2-formyl phenylboronic acid†
Abstract
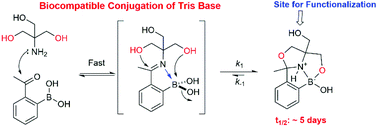
We describe the biocompatible conjugation of the Tris base to 2-formyl and 2-acetylphenylboronic acid (abbreviated as 2-FPBA and 2-APBA respectively), which have emerged as a versatile chemotype for fast biocompatible conjugation reactions. Tris base was found to react with 2-FPBA/APBA to give oxazolidinoboronate (OzB) complexes, analogous to the thiazolidinoboronate (TzB) and imidazolidinoboronate (IzB) complex formation that we recently reported. The Tris conjugations proceed well in complex biological media, and in contrast to the TzB/IzB complexes, the Tris conjugates exhibit superior kinetic stability (dissociation over days) as well as chemical stability against oxidation. We demonstrate the utility of such conjugation chemistries via a small molecule-induced peptide cyclization in blood serum.
- This article is part of the themed collection: Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...