Amine-tethered phenylboronic acid-enabling ring-opening strategy for carbon chain elongation from double aldol cyclic hemiacetals†
Abstract
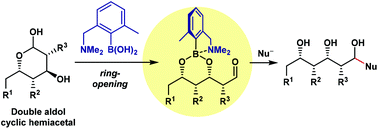
The addition of carbon nucleophiles to cyclic hemiacetal forms of double aldols is a promising approach toward the synthesis of structurally attractive 1,3-polyol derivatives. Cyclic hemiacetals are generally unreactive to carbon nucleophiles under neutral conditions, however, because the electrophilic aldehyde function is masked. Here we developed an amine-tethered phenylboronic acid 7g, which transforms double aldol cyclic hemiacetals to ring-opened linear aldehydes. Combined with the previously-developed copper-catalysed asymmetric double aldol reaction (L. Lin, K. Yamamoto, H. Mitsunuma, Y. Kanzaki, S. Matsunaga and M. Kanai, J. Am. Chem. Soc., 2015, 137, 15418), this method produced synthetically useful chiral building blocks containing a 1,3-di- or tri-ol moiety.
- This article is part of the themed collections: Synthetic methodology in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...