Two-component boronic acid catalysis for increased reactivity in challenging Friedel–Crafts alkylations with deactivated benzylic alcohols†
Abstract
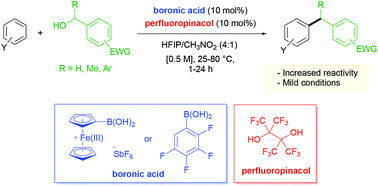
A general and efficient boronic acid catalyzed Friedel–Crafts alkylation of arenes with benzylic alcohols was previously developed for the construction of unsymmetrical diarylmethane products (X. Mo, J. Yakiwchuk, J. Dansereau, J. A. McCubbin and D. G. Hall, J. Am. Chem. Soc., 2015, 137, 9694). Highly electron-deficient benzylic alcohols, however, were ineffective coupling partners due to the increased difficulty of C–O bond ionization. Herein, we report the use of perfluoropinacol as an effective co-catalyst to improve the reactivity of a boronic acid catalyst in the Friedel–Crafts benzylations of electronically deactivated primary and secondary benzylic alcohols. According to spectroscopic studies, it is believed that perfluoropinacol condenses with the arylboronic acid catalyst to form a highly electrophilic and Lewis acidic boronic ester. This in situ formed species enables a more facile ionization of the benzylic alcohols likely through a mode of activation promoted by a Lewis acid assisted hydronium Brønsted acid generated from the interactions of the transient boronic ester with hexafluoroisopropanol solvent and water.
- This article is part of the themed collections: Synthetic methodology in OBC and Trends in Organoboron Chemistry


 Please wait while we load your content...
Please wait while we load your content...