Themed collection Natural product synthesis

Recent development on the [5+2] cycloadditions and their application in natural product synthesis
The recent developments on the [5+2] cycloadditions and their application in the synthesis of complex natural products are discussed.
![Graphical abstract: Recent development on the [5+2] cycloadditions and their application in natural product synthesis](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C8CC09077G&imageInfo.ImageIdentifier.Year=2019)
Chem. Commun., 2019,55, 1859-1878
https://doi.org/10.1039/C8CC09077G
Regioselective side-chain amination of 2-alkyl azacycles by radical translocation: total synthesis of tetraponerine T8
Radical translocation facilitates the regioselective γ-amination of 2-alkyl-substituted azacycles, leading to 1,3-diamines including the alkaloidal natural product tetraponerine T8.

Chem. Commun., 2021,57, 919-922
https://doi.org/10.1039/D0CC07625B
Access to 1-indolyltetrahydro-β-carbolines via metal-free cross-dehydrogenative coupling: the total synthesis of eudistomin U, isoeudistomin U and 19-bromoisoeudistomin U
A highly selective metal-free CDC approach for the synthesis of 1-indolyltetrahydro-β-carbolines is developed. The total synthesis of biologically important alkaloids, eudistomin U and isoeudistomin U and the first total synthesis of 19-bromoisoeudistomin U were accomplished.
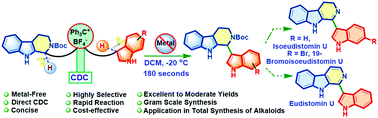
Chem. Commun., 2021,57, 757-760
https://doi.org/10.1039/D0CC06958B
Collective enantioselective total synthesis of (+)-sinensilactam A, (+)-lingzhilactone B and (−)-lingzhiol: divergent reactivity of styrene
The collective total synthesis of (+)-sinensilactam A, (+)-lingzhilactone B, (+)-lingzhilactone C and (−)-lingzhiol has been accomplished from a common epoxide intermediate 9.

Chem. Commun., 2020,56, 10066-10069
https://doi.org/10.1039/D0CC04064A
A macrocycle directed total synthesis of di-O-methylendiandrin A
The synthesis of di-O-methylendiandrin A has been achieved using diastereoselective, vicinal alkylation and transannular McMurry reactions of a macrocyclic 1,4-diketone, to establish stereochemistry and the strained ring of the natural product.
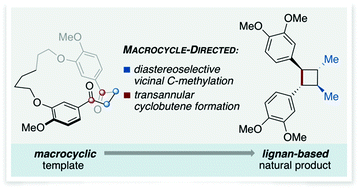
Chem. Commun., 2020,56, 8747-8749
https://doi.org/10.1039/D0CC03302B
Chemoenzymatic, biomimetic total synthesis of (−)-rugulosin B, C and rugulin analogues and their biosynthetic implications
Flavoskyrins, (−)-rugulosin B, C and rugulin analogues are synthesized chemoenzymatically from anthraquinones in two, three and four steps, respectively.
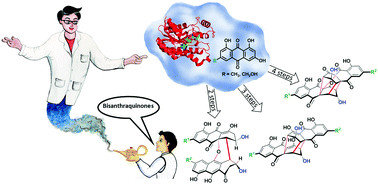
Chem. Commun., 2020,56, 3337-3340
https://doi.org/10.1039/D0CC00406E
Total synthesis and biological evaluation of simplified aplyronine analogues as synthetically tractable anticancer agents
Analysis of bound structures and SAR studies led to the function-oriented simplification of the aplyronines; a convergent and modular synthetic platform then enabled the total synthesis and biological evaluation of four novel analogues.
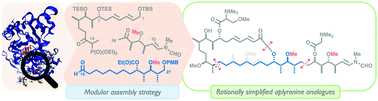
Chem. Commun., 2020,56, 1529-1532
https://doi.org/10.1039/C9CC09050A
Exploration of a KI-catalyzed oxidation system for direct construction of bispyrrolidino[2,3-b]indolines and the total synthesis of (+)-WIN 64821
A facile and environmentally benign KI(cat.)/NaBO3·4H2O oxidation system has been developed for the tandem oxidative aminocyclization/coupling of tryptamines, affording a series of 3a,3a′-bispyrrolidino[2,3-b]indolines with high efficiency (up to 94% yield).
![Graphical abstract: Exploration of a KI-catalyzed oxidation system for direct construction of bispyrrolidino[2,3-b]indolines and the total synthesis of (+)-WIN 64821](/en/Image/Get?imageInfo.ImageType=GA&imageInfo.ImageIdentifier.ManuscriptID=C9CC08646C&imageInfo.ImageIdentifier.Year=2020)
Chem. Commun., 2020,56, 121-124
https://doi.org/10.1039/C9CC08646C
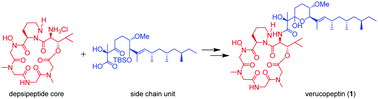
Total synthesis of verucopeptin, an inhibitor of hypoxia-inducible factor 1 (HIF-1)
The first total synthesis of vercopeptin (1), an inhibitor of hypoxia-inducible factor 1 (HIF-1), was achieved via condensation of the depsipeptide core and the polyketide side chain unit.
Chem. Commun., 2019,55, 11956-11959
https://doi.org/10.1039/C9CC06169J
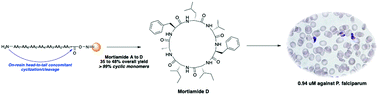
Total synthesis and antimalarial activity of mortiamides A–D
This work describes the first total synthesis of mortiamides and their anti-malarial activity against a multi-drug resistant strain of Plasmodium falciparum.
Chem. Commun., 2019,55, 7434-7437
https://doi.org/10.1039/C9CC02864A
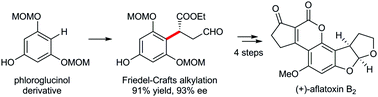
Organocatalytic enantioselective direct alkylation of phloroglucinol derivatives: asymmetric total synthesis of (+)-aflatoxin B2
The organocatalytic enantioselective Friedel–Crafts alkylation of the phloroglucinol derivatives with enals is reported, providing general access to the benzylic chiral centers shown in a variety of phloroglucinol natural products.
Chem. Commun., 2019,55, 5171-5174
https://doi.org/10.1039/C9CC01833F
A synthetic approach to chrysophaentin F
A synthetic approach to chrysophaentin F is described featuring an array of metal catalysed coupling reactions (Cu, Ni, Pd, W, Mo).

Chem. Commun., 2019,55, 4837-4840
https://doi.org/10.1039/C9CC01666J
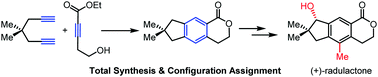
Divergent total syntheses of five illudalane sesquiterpenes and assignment of the absolute configuration
Transition metal catalyzed [2+2+2] cycloaddition and aromatic C–H oxygenation enables concise, divergent total syntheses of five bioactive illudalane sesquiterpenes.
Chem. Commun., 2019,55, 4250-4253
https://doi.org/10.1039/C9CC00933G
First total synthesis of ent-asperparaline C and assignment of the absolute configuration of asperparaline C
The first asymmetric total synthesis of a member of the asperparaline family was accomplished and the unknown absolute configuration of asperparaline C has been determined to be all-(S).
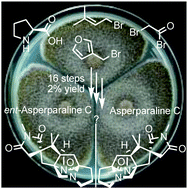
Chem. Commun., 2019,55, 3931-3934
https://doi.org/10.1039/C9CC00945K
Unified enantioselective total syntheses of (−)-scholarisine G, (+)-melodinine E, (−)-leuconoxine and (−)-mersicarpine
A unified strategy enabled the enantioselective syntheses of (−)-scholarisine G, (+)-melodinine E, (−)-leuconoxine and (−)-mersicarpine from a common 2-alkylated indole bearing an all-carbon quaternary stereogenic center.

Chem. Commun., 2019,55, 3544-3547
https://doi.org/10.1039/C8CC09949A
A concise synthesis and biological study of evodiamine and its analogues
Efficient access to evodiamine and its analogues is presented via Lewis acid catalysis. In this reaction, three chemical bonds and two heterocyclic-fused rings are constructed in one step.
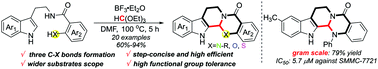
Chem. Commun., 2019,55, 3089-3092
https://doi.org/10.1039/C9CC00434C
A desymmetrization-based approach to morphinans: application in the total synthesis of oxycodone
Here we report a total synthesis of the pharmacologically significant morphinan alkaloid, oxycodone.
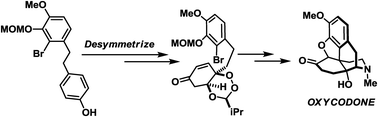
Chem. Commun., 2018,54, 13018-13021
https://doi.org/10.1039/C8CC07667G
Catalytic stereoselective total synthesis of a spiro-oxindole alkaloid and the pentacyclic core of tryptoquivalines
An expedient route to the pentacyclic core of the tryptoquivaline alkaloids and the total synthesis of natural product (+)-3′-(4-oxoquinazolin-3-yl)spiro[1H-indole-3,5′-oxolane]-2,2′-dione (1) have been achieved.
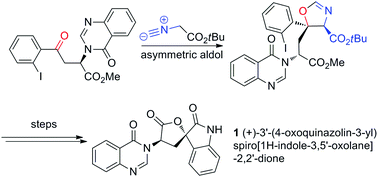
Chem. Commun., 2018,54, 12860-12862
https://doi.org/10.1039/C8CC07479H
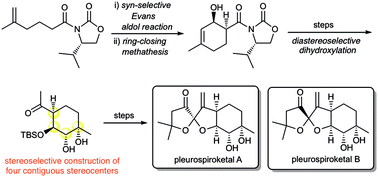
Asymmetric total synthesis of pleurospiroketals A and B
The first asymmetric total synthesis of pleurospiroketals A and B has been accomplished in 16 steps from 5-methyl-5-hexenoic acid.
Chem. Commun., 2018,54, 10316-10319
https://doi.org/10.1039/C8CC06185H
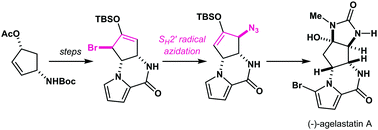
Total synthesis of (−)-agelastatin A: an SH2′ radical azidation strategy
A new synthetic approach to (−)-agelastatin A has been established through the strategic implementation of brominative olefin transposition of a silyl enol ether and subsequent SH2′ radical azidation of the resultant allylic bromide.
Chem. Commun., 2018,54, 9893-9896
https://doi.org/10.1039/C8CC05697H
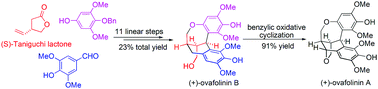
Asymmetric total synthesis of (+)-ovafolinins A and B
Benefiting from a highly diastereoselective alkylation of (S)-Taniguchi lactone, a double Friedel–Crafts reaction, a global debenzylation and a Cu(OAc)2-enabled benzylic oxidative cyclization, an efficient synthetic approach to (+)-ovafolinins A and B has been developed.
Chem. Commun., 2018,54, 7539-7541
https://doi.org/10.1039/C8CC03456G
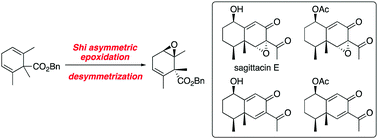
Enantioselective total synthesis of sagittacin E and related natural products
The first enantioselective total synthesis of eremophilane-type sesquiterpenoids, sagittacin E and related natural products, was achieved.
Chem. Commun., 2018,54, 6165-6168
https://doi.org/10.1039/C8CC03438A
Alkene protection against acid using a bromide substituent: application in a total synthesis of (−)-6,7-dideoxysqualestatin H5
Alkenyl bromide functionality survives several synthetic steps, including an acid-induced rearrangement, before a stereoretentive methylative debromination gives the natural product.
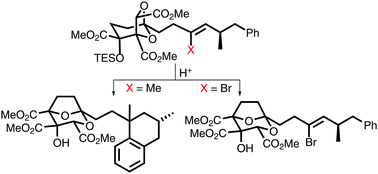
Chem. Commun., 2018,54, 5354-5356
https://doi.org/10.1039/C8CC02690D
A divergent and concise total synthesis of (−)-lycoposerramine R and (+)-lycopladine A
A concise, asymmetric and divergent synthesis of lycoposerramine R and lycopladine A is presented.
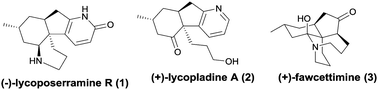
Chem. Commun., 2018,54, 3598-3600
https://doi.org/10.1039/C8CC01626G
Nine-step total synthesis of (−)-strychnofoline
The asymmetric synthesis of an anti-cancer spirooxindole alkaloid, (−)-strychnofoline, has, for the first time, been accomplished in only nine steps.
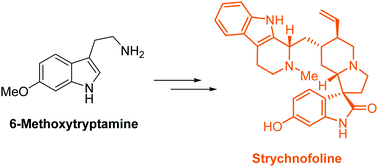
Chem. Commun., 2018,54, 1125-1128
https://doi.org/10.1039/C7CC08938D
Total synthesis of conosilane A via a site-selective C–H functionalization strategy
The strategy developed for the first total synthesis of highly oxygenated natural product conosilane A involving double manipulation of allylic C(sp3)–H functionalization renders the power of C–H functionalization in organic syntheses.
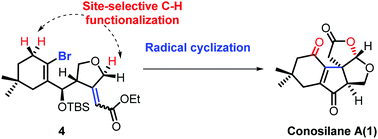
Chem. Commun., 2018,54, 912-915
https://doi.org/10.1039/C7CC09367E
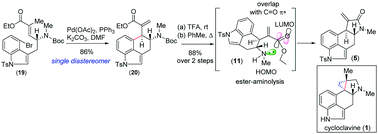
Catalytic asymmetric formal total syntheses of (+)- and (−)-cycloclavine
Catalytic asymmetric formal syntheses of both enantiomers of (+)- and (−)-cycloclavine (1) have been envisioned via proline catalysed α-aminoxylation of aldehydes followed by an intramolecular Heck cyclization to set vicinal stereocenters.
Chem. Commun., 2018,54, 940-943
https://doi.org/10.1039/C7CC09045E
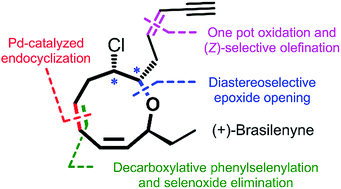
Total synthesis of (+)-brasilenyne via concise construction of an oxonane framework containing a 1,3-cis,cis-diene
The enantioselective total synthesis of (+)-brasilenyne, which has a unique oxonane framework containing a 1,3-cis,cis-diene, has been accomplished.
Chem. Commun., 2018,54, 467-470
https://doi.org/10.1039/C7CC08329G
About this collection
Collated by Antonio Echavarren (ICIQ, Spain), this collection showcases syntheses designed as a proof of the applicability of a new synthetic methods as well as for the obvious purpose of accessing to biologically relevant molecules, although in occasions these synthesis still fulfill the traditional objective of definitively assigning the structure of the natural product.