A macrocycle directed total synthesis of di-O-methylendiandrin A†
Abstract
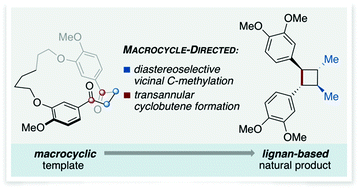
The total synthesis of the lignan-based cyclobutane di-O-methylendiandrin A has been achieved using diastereoselective, vicinal alkylation and transannular McMurry reactions of a macrocyclic 1,4-diketone as key transformations for establishing relative stereochemistry and furnishing the strained 4-membered ring of the natural product.
- This article is part of the themed collections: 2020 Emerging Investigators and Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...