Catalytic stereoselective total synthesis of a spiro-oxindole alkaloid and the pentacyclic core of tryptoquivalines†
Abstract
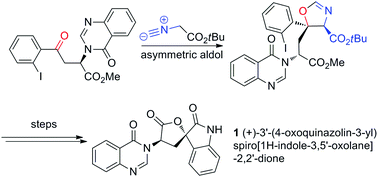
An expedient route to the pentacyclic core of the tryptoquivaline alkaloids and the total synthesis of natural product (+)-3′-(4-oxoquinazolin-3-yl)spiro[1H-indole-3,5′-oxolane]-2,2′-dione (1) have been achieved. The route is enabled by a key, highly stereoselective, aldol reaction catalysed by a Ag(I) and cinchona-derived aminophosphine ligand system, forming a highly substituted oxazoline ring, and setting the C1 spirocyclic stereocentre for downstream manipulation.
- This article is part of the themed collection: Natural product synthesis


 Please wait while we load your content...
Please wait while we load your content...