Total synthesis of (−)-agelastatin A: an SH2′ radical azidation strategy†
Abstract
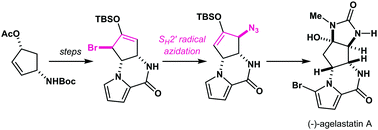
A reagent generated from TMSN3/KMnO4/BnEt3NCl was found to promote an SH2′ radical azidation of a bromo silyl enol ether to furnish an azido silyl enol ether via olefin transposition. With the present azidation protocol, a new synthetic approach to agelastatin A, a potent antitumor marine alkaloid, has been established.
- This article is part of the themed collection: Natural product synthesis

 Please wait while we load your content...
Please wait while we load your content...