Themed collection Editor’s Choice – Ning Jiao

Electro-organic synthesis – a 21st century technique
This perspective provides insight into recent electro-organic methods and general trends in this field, and opens up prospects for future viewpoints.
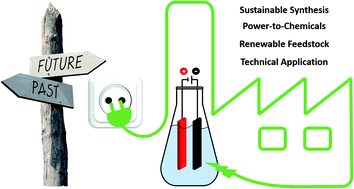
Chem. Sci., 2020,11, 12386-12400
https://doi.org/10.1039/D0SC01848A
Tuning the stability of organic radicals: from covalent approaches to non-covalent approaches
Covalent and non-covalent approaches to tune the stability of organic radicals through steric effects and the delocalization of spin density.
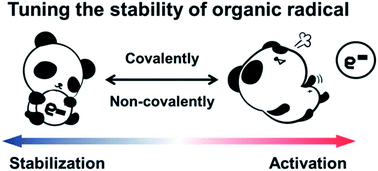
Chem. Sci., 2020,11, 1192-1204
https://doi.org/10.1039/C9SC06143F
A happy medium: the synthesis of medicinally important medium-sized rings via ring expansion
Ring expansion strategies are ideally suited to make synthetically challenging, medium-sized rings with much potential in medicinal chemistry.
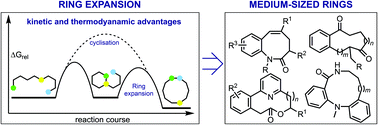
Chem. Sci., 2020,11, 2876-2881
https://doi.org/10.1039/D0SC00568A
Silyloxymethanesulfinate as a sulfoxylate equivalent for the modular synthesis of sulfones and sulfonyl derivatives
An efficient protocol for the modular synthesis of sulfones and sulfonyl derivatives has been developed utilizing sodium tert-butyldimethylsilyloxymethanesulfinate (TBSOMS-Na) as a sulfoxylate (SO22−) equivalent.
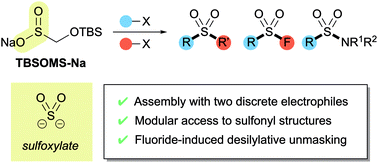
Chem. Sci., 2020,11, 13071-13078
https://doi.org/10.1039/D0SC02947E
A copper-catalyzed asymmetric oxime propargylation enables the synthesis of the gliovirin tetrahydro-1,2-oxazine core
The bicyclic tetrahydro-1,2-oxazine subunit of gliovirin is synthesized through a diastereoselective copper-catalyzed cyclization of an N-hydroxyamino ester.

Chem. Sci., 2020,11, 11897-11901
https://doi.org/10.1039/D0SC04802J
Total synthesis of biseokeaniamides A–C and late-stage electrochemically-enabled peptide analogue synthesis
A late-stage electrochemical decarboxylation enables rapid access to structural analogues of biseokeaniamides A–C, cytotoxic lipopeptide natural products.
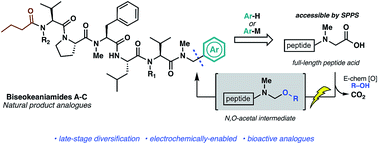
Chem. Sci., 2020,11, 10752-10758
https://doi.org/10.1039/D0SC03701J
Peptide late-stage C(sp3)–H arylation by native asparagine assistance without exogenous directing groups
An efficient method for peptide late-stage C(sp3)-H arylations assisted by unmodified side chain of asparagine (Asn) without any exogenous directing group has been reported.
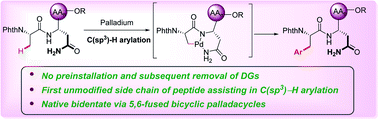
Chem. Sci., 2020,11, 9290-9295
https://doi.org/10.1039/D0SC03830J
Programmable synthesis of multiply arylated cubanes through C–H metalation and arylation
Cubane has attracted attention due to its unique 3D structure. Herein, we report the programmable synthesis of multiply arylated cubanes. The developed reaction allows the late-stage and regioselective installation of aryl groups.
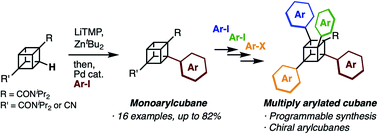
Chem. Sci., 2020,11, 7672-7675
https://doi.org/10.1039/D0SC01909G
Palladium-catalyzed dearomative 1,4-difunctionalization of naphthalenes
The dearomative 1,4-difunctionalization of naphthalenes is achieved by imitating the reactivity of simple conjugated dienes in aromatic systems, providing functionalized spirooxindoles in high yields (up to 99%) with exclusive diastereoselectivity.
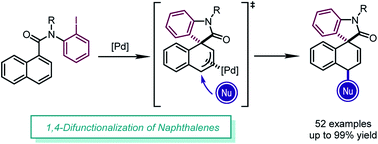
Chem. Sci., 2020,11, 6830-6835
https://doi.org/10.1039/D0SC02816A
Transferring axial molecular chirality through a sequence of on-surface reactions
The axial chirality of reactants is transferred through multistep on-surface reactions to chiral polymers and to prochiral graphene nanoribbons.
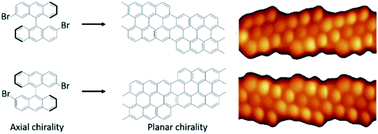
Chem. Sci., 2020,11, 5441-5446
https://doi.org/10.1039/D0SC01653E
Facile access to diverse all-carbon quaternary center containing spirobicycles by exploring a tandem Castro–Stephens coupling/acyloxy shift/cyclization/semipinacol rearrangement sequence
An efficient construction of spirocyclo[4.5]decane derivatives is developed via a Castro–Stephens coupling/1,3-acyloxy shift/cyclization/semipinacol rearrangement sequence.
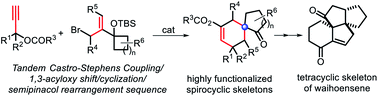
Chem. Sci., 2020,11, 3878-3884
https://doi.org/10.1039/D0SC00102C
Sulfoxide-mediated oxidative cross-coupling of phenols
A metal-free, oxidative coupling of phenols with various nucleophiles, including arenes, 1,3-diketones and other phenols, is reported.

Chem. Sci., 2020,11, 2001-2005
https://doi.org/10.1039/C9SC05668H
Lipshutz-type bis(amido)argentates for directed ortho argentation
Bis(amido)argentate (TMP)2Ag(CN)Li2 was designed as a tool for highly chemoselective aromatic functionalization via unprecedented directed ortho argentation (DoAg).
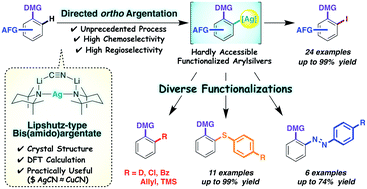
Chem. Sci., 2020,11, 1855-1861
https://doi.org/10.1039/C9SC06060J
Controllable double CF2-insertion into sp2 C–Cu bond using TMSCF3: a facile access to tetrafluoroethylene-bridged structures
Copper-mediated controllable double CF2 insertion into sp2 C–Cu bond and subsequent cross-coupling reaction open the door to the synthesis of an array of valuable tetrafluoroethylene-bridged molecules.
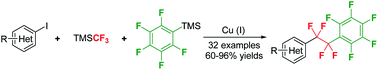
Chem. Sci., 2020,11, 276-280
https://doi.org/10.1039/C9SC05018C
Catalytic, transannular carbonyl-olefin metathesis reactions
Transannular carbonyl-olefin metathesis reactions complement existing procedures for related ring-closing, ring-opening, and intermolecular carbonyl-olefin metathesis. This enables molecular editing of steroid-derived frameworks.
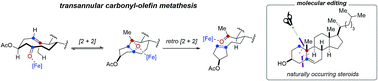
Chem. Sci., 2019,10, 10267-10274
https://doi.org/10.1039/C9SC03716K
Saturated oxygen and nitrogen heterocycles via oxidative coupling of alkyltrifluoroborates with alkenols, alkenoic acids and protected alkenylamines
The oxidative coupling of alkyltrifluoroborates with heteroatom-tethered vinylarenes leads to a broad range of saturated oxygen and nitrogen heterocycles.
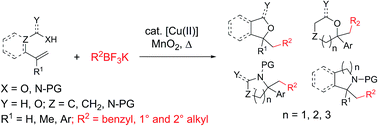
Chem. Sci., 2019,10, 9265-9269
https://doi.org/10.1039/C9SC02835H
Oxidant speciation and anionic ligand effects in the gold-catalyzed oxidative coupling of arenes and alkynes
The mechanism of the gold-catalyzed oxidative cross-coupling of arenes and alkynes has been studied in detail combining stoichiometric experiments with putative reaction intermediates and DFT calculations.

Chem. Sci., 2019,10, 8411-8420
https://doi.org/10.1039/C9SC02372K
S8-Catalyzed triple cleavage of bromodifluoro compounds for the assembly of N-containing heterocycles
An unprecedented S8-catalyzed selective triple-cleavage of bromodifluoroacetamides is disclosed for the first time.
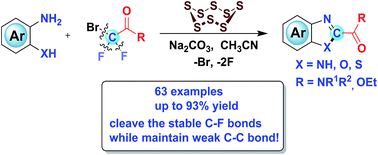
Chem. Sci., 2019,10, 6828-6833
https://doi.org/10.1039/C9SC01333D
Direct conversion of phenols into primary anilines with hydrazine catalyzed by palladium
A general and practical method to directly convert phenols into primary anilines with cheap and easy-to-handle hydrazine as the amine and hydride sources catalyzed by Pd/C.

Chem. Sci., 2019,10, 4775-4781
https://doi.org/10.1039/C9SC00595A
Aromatic C–H amination in hexafluoroisopropanol
We report direct amination of electron-poor arenes and evaluate the crucial factors for the enhanced reactivity in hexafluoroisopropanol.

Chem. Sci., 2019,10, 2424-2428
https://doi.org/10.1039/C8SC04966A
About this collection
In 2021, Chemical Science welcomed Professor Ning Jiao to the journal as an Associate Editor, handling papers in the area of organic chemistry. Ning has looked back over recent Chemical Science papers and has selected some outstanding articles that he would like to share, including research highlighting optimised total synthesis methodology, cross coupling reactions, and electro-organic synthesis. We hope you enjoy reading through this selection.