Facile access to diverse all-carbon quaternary center containing spirobicycles by exploring a tandem Castro–Stephens coupling/acyloxy shift/cyclization/semipinacol rearrangement sequence†
Abstract
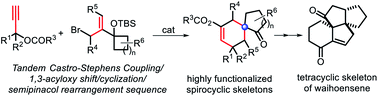
Efficient combination of two or more reactions into a practically useful purification free sequence is of great significance for the achievement of structural complexity and diversity, and an important approach for the development of new synthetic strategies that are industrially step-economic and environmentally friendly. In this work, a facile and efficient method for the construction of highly functionalized spirocyclo[4.5]decane derivatives containing a synthetically challenging quaternary carbon center has been successfully developed through the realization of a tandem Castro–Stephens coupling/1,3-acyloxy shift/cyclization/semipinacol rearrangement sequence. Thus a series of multi-substituted spirocyclo[4.5]decane and functionalized cyclohexane skeletons with a phenyl-substituted quaternary carbon center have been constructed using this method as illustrated by 24 examples in moderate to good yields. The major advantages of this method over the known strategies are better transformation efficiency (four consecutive transformations in one tandem reaction), product complexity and diversity. As a support of its potential application, a quick construction of the key tetracyclic diterpene skeleton of waihoensene has been achieved.
- This article is part of the themed collection: Editor’s Choice – Ning Jiao


 Please wait while we load your content...
Please wait while we load your content...