Palladium-catalyzed dearomative 1,4-difunctionalization of naphthalenes†
Abstract
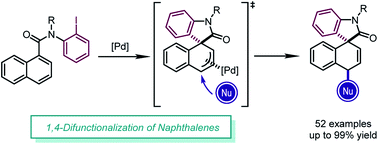
A highly diastereoselective dearomatization of naphthalenes via a Pd-catalyzed 1,4-difunctionalization reaction is described. In the presence of a commercially available palladium precursor and ligand, intramolecular dearomative Heck-type insertion provides π-allylpalladium intermediates which are readily captured by a series of nucleophiles in excellent yields (up to 99%). This reaction features mild conditions, broad substrate scope, and useful transformations of the products.
- This article is part of the themed collection: Editor’s Choice – Ning Jiao


 Please wait while we load your content...
Please wait while we load your content...