Open Access Article
Open Access ArticleFacile access to diverse all-carbon quaternary center containing spirobicycles by exploring a tandem Castro–Stephens coupling/acyloxy shift/cyclization/semipinacol rearrangement sequence†
Ye
Zhang
a,
Tian-Lu
Zheng
a,
Fu
Cheng
a,
Kun-Long
Dai
a,
Kun
Zhang
b,
Ai-Jun
Ma
b,
Fu-Min
Zhang
 a,
Xiao-Ming
Zhang
a,
Xiao-Ming
Zhang
 a,
Shao-Hua
Wang
a,
Shao-Hua
Wang
 *ab and
Yong-Qiang
Tu
*ab and
Yong-Qiang
Tu
 *ac
*ac
aState Key Laboratory of Applied Organic Chemistry, School of Pharmacy, Lanzhou University, Lanzhou 730000, P. R. China. E-mail: tuyq@lzu.edu.cn; wangshh@lzu.edu.cn
bSchool of Biotechnology and Health Science, Wuyi University, Jiangmen 529020, P. R. China
cSchool of Chemistry and Chemical Engineering, Shanghai Key Laboratory for Molecular Engineering of Chiral Drugs, Shanghai Jiao Tong University, Shanghai 200240, P. R. China
First published on 16th March 2020
Abstract
Efficient combination of two or more reactions into a practically useful purification free sequence is of great significance for the achievement of structural complexity and diversity, and an important approach for the development of new synthetic strategies that are industrially step-economic and environmentally friendly. In this work, a facile and efficient method for the construction of highly functionalized spirocyclo[4.5]decane derivatives containing a synthetically challenging quaternary carbon center has been successfully developed through the realization of a tandem Castro–Stephens coupling/1,3-acyloxy shift/cyclization/semipinacol rearrangement sequence. Thus a series of multi-substituted spirocyclo[4.5]decane and functionalized cyclohexane skeletons with a phenyl-substituted quaternary carbon center have been constructed using this method as illustrated by 24 examples in moderate to good yields. The major advantages of this method over the known strategies are better transformation efficiency (four consecutive transformations in one tandem reaction), product complexity and diversity. As a support of its potential application, a quick construction of the key tetracyclic diterpene skeleton of waihoensene has been achieved.
Introduction
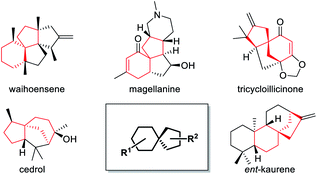
Spiro bicyclic scaffolds incorporating all-carbon quaternary stereocenters, as a big number of key structural moieties, broadly exist in bioactive natural products and pharmaceutical molecules, and often play essential roles in their characteristic bioactivity.1 Because of the highly steric repulsion caused by its four carbonic substituents,2 however, the construction of this type of moiety is a long-standing challenging topic.3 Taking the spirocyclo[4.5]decane skeleton as a typical example, a lot of bioactive natural products as well as synthetic intermediates contain this key unit (Fig. 1).4 Therefore, in order to synthesize these target molecules and facilitate corresponding molecular function studies, how to efficiently construct this unit has become a crucial and difficult step. Although several strategies based on different intermolecular or intramolecular cycloaddition patterns of relatively complex substrates have been developed during the past decade,5 it is still highly desirable to further explore alternative approaches for pursuing transformation efficiency, and product diversity as well as extensibility.Results
Design plan
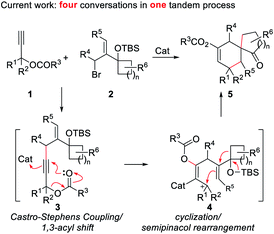
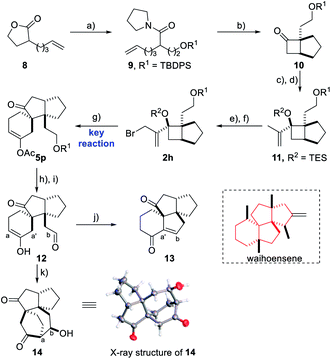
Besides the above strategies, it is particularly noticeable that the semipinacol rearrangement reaction, which can generate a spirocyclic quaternary carbon center through functional group migration or skeleton reorganization, has been used by us6 and several other research groups7 for the syntheses of a variety of bioactive molecules containing spirocyclo[4.5]decane and related skeletons. Accordingly, the development of more efficient semipinacol rearrangement involved reaction patterns is always a main research program of our group, especially, through ingenious design and realization of sequential chemical transformations in step economy. Inspired by the special chemical properties of propargyl ester in acyloxy shift/cyclizations,8 we envisioned that a 1,3-acyloxy shift of the propargyl ester9 intermediate 3 generated by a Castro–Stephens coupling10 from propargyl ester 1 and allylic bromide 2 possessing a potential migration moiety might be viable to trigger a cyclization/semipinacol rearrangement of 4 affording multi-substituted spirocyclo[4.5]decane and related skeletons (Scheme 1). Based on the mechanism analysis of the above four transformations, it is highly likely to achieve them in a tandem manner. Additionally, since a series of substituent combinations can be used from the two substrates, this strategy, once feasible, will not only exhibit better transformation efficiency and product complexity and diversity, but also further enrich the content of semipinacol rearrangement. Herein, we present such a novel sequence and its application in the construction of the tetracyclic ring system of waihoensene, a new example of complex bioactive molecule-directed synthetic methodology development.Discussion
Optimization of reaction conditions
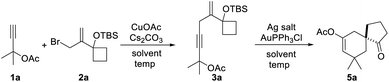
Following the above assumption, we first tested the feasibility of the designed tandem reaction using propargyl ester 1a and allylic bromide 2a as the model substrates. As the copper catalyst has been successfully applied in Castro–Stephens coupling and the 1,3-acyloxy shift process to form an allene group,11 different copper catalysts were examined for promoting the expected reaction.12 Although none of them afforded the desired final product 5a, most could give the Castro–Stephens coupling product 3a, with a best yield of 68% using CuOAc.12 Further screening of the base additive, solvent and reaction temperature showed that the use of Cs2CO3 in DCE at 70 °C could produce 3a in the best yield of 82% (Table 1, entry 1). Subsequently, the realization of the initial tandem reaction in a purification free manner was then investigated. Fortunately, after a quick removal of the solid from the reaction mixture through Celite pad filtration following the Castro–Stephens coupling reaction, a first attempt using the combination of 10 mol% AuPPh3Cl and AgOTf could promote the desired reaction to give the product 5a in 57% yield (Table 1, entry 7). Furthermore, the counterion effect13 was also observed with this reaction, and the use of counterion NTf2− from AgNTf2 exhibited the best result of 66% yield for 5a (Table 1, entry 8). Next, several different solvents were applied to this tandem reaction. Among them, benzene, THF and CH3CN could give the desired product in low yield (Table 1, entries 11–16). Other solvents were not compatible with this transformation. Finally, the use of CuOAc along with Cs2CO3 and the combination of AuPPh3Cl and AgNTf2 in DCE (Table 1, entry 8) was selected as the optimal reaction conditions.| Entry | Solvent | Ag salt | Temp | Product | Yield |
|---|---|---|---|---|---|
| a Unless specified, all reactions were carried out using 1a (0.5 mmol, 2.5 eq.), 2a (0.2 mmol, 1.0 eq.), CuOAc (30 mol%), Cs2CO3 (50 mol%), AuPPh3Cl (10 mol%), and Ag salt (10 mol%) in a reaction tube in DCE (2 mL) at indicated temperature. b The solvent of Castro–Stephens coupling for 3a. c Temperature for the first coupling reaction. d Isolated yield of 3a. e The first coupling step was carried out at 70 °C. f Isolated yield of 5a in a purification free manner. g After filtration, the filtrate was concentrated and diluted with the indicated solvent (4 mL) for the subsequent operation. | |||||
| 1 | DCEb | — | 70 °Cc | 3a | 82%d |
| 2 | Benzeneb | — | rtc | 3a | 40%d |
| 3 | THFb | — | rtc | 3a | 47%d |
| 4 | EtOHb | — | rtc | 3a | 55%d |
| 5 | CH3CNb | — | rtc | 3a | 55%d |
| 6 | DMFb | — | rtc | 3a | 54%d |
| 7 | DCE | AgOTf | rte | 5a | 57%f |
| 8 | DCE | AgNTf2 | rte | 5a | 66%f |
| 9 | DCE | AgSbF6 | rte | 5a | 53%f |
| 10 | DCE | AgBF4 | rte | 5a | 49%f |
| 11 | Benzeneg | AgNTf2 | rte | 5a | 11%f |
| 12 | THFg | AgNTf2 | rte | 5a | 27%f |
| 13 | EtOHg | AgNTf2 | rte | 5a | nd |
| 14 | CH3CNg | AgNTf2 | rte | 5a | 53%f |
| 15 | DMFg | AgNTf2 | rte | 5a | nd |
| 16 | DMSOg | AgNTf2 | rte | 5a | nd |
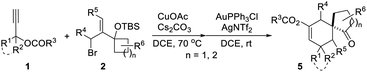
Substrate scope investigation
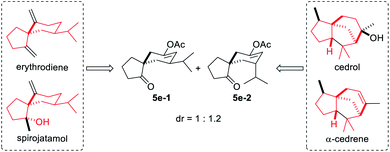
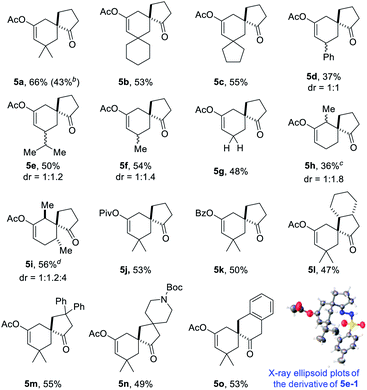
With the optimal reaction conditions in hand (Table 1, entry 8), we began to explore the generality of this reaction, and the results are summarized in Tables 2 and 3. Among the substrates tested, most of them could give the expected products in moderate to good yields. When R1 and R2 groups of propargyl ester formed a ring system (i.e., cyclohexyl and cyclopentyl), the reaction with bromide 2a (R4, R5, R6 = H) proceeded smoothly providing tricyclic products 5b and 5c in 53% and 55% yield, respectively. Moreover, with the R1 as H, R2 could be H, Ph, isopropyl, or methyl, and all reactions could give the desired products 5d to 5g in moderate to good yields, albeit with low diastereoselectivity for 5d to 5f. Additionally, the reaction was also compatible with substrate 2 with different R4/R5 groups. In the case of substrate 2b with R4 and R5 being Me and H, respectively, the expected product 5h was obtained in 36% yield and 1/1 dr ratio. When both R4 and R5 were Me (2c), the desired product 5i was produced with good diastereoselectivity and yield. It should be noted that a series of natural product skeletons might be obtained by our protocol. For example, since the relative configuration of the two diastereoisomers of ketone 5e (ref. 14) (5e-1 and 5e-2) is consistent with natural products erythrodiene4c,d and cedrol,4g,h respectively, a new general synthetic strategy for these types of natural products might be developed based on propargyl ester with pivaloyl, and benzoyl was successful to give the corresponding products 5j and 5k in 53% and 50% yield, respectively. In order to prove the efficiency of this method in the rapid construction of product complexity, other four allylic bromides 2d–2g were applied to the reaction, which produced four spirocyclic products in good yields. Among them, products 5l and 5m confirmed the feasibility of adding additional substituents on the cyclobutanol moiety, while product 5n showed that the amine group is amenable to this reaction. It was noteworthy that substrate 2g with a 2,3-dihydro-1H-inden-2-ol motif could go through the reaction through a five-membered ring to a six-membered ring expansion affording product 5o. In order to demonstrate the potential utility of such a reaction, the transformation between 1a and 2a was attempted on the gram scale giving 5a in a moderate yield of 43% (Scheme 2).| a Unless specified, all reactions were conducted using 1 (0.5 mmol, 2.5 eq.), 2 (0.2 mmol, 1.0 eq.), CuOAc (30 mol%), Cs2CO3 (50 mol%), AuPPh3Cl (10 mol%), and AgNTf2 (10 mol%) in a reaction tube in DCE (2 mL) at indicated temperature. b 10 mmol of 1a (1.26 g) and 4 mmol of 2a (1.22 g) were used. c CHCl3 was used as the solvent after Castro–Stephens coupling. d AuCl3 (10 mol%) in 2 mL HFB (hexafluorobenzene) was used instead of the combination of AuPPh3Cl and AgNTf2 after Castro–Stephens coupling; the structure shows the relative configuration of the major isomer. |
|---|
|
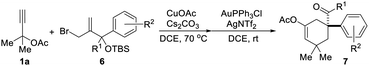
| a All reactions were conducted using 1a (0.5 mmol, 2.5 eq.), 6 (0.2 mmol, 1.0 eq.), CuOAc (30 mol%), Cs2CO3 (50 mol%), AuPPh3Cl (10 mol%), and AgNTf2 (10 mol%) in a reaction tube in DCE (2 mL) at indicated temperature. |
|---|
|
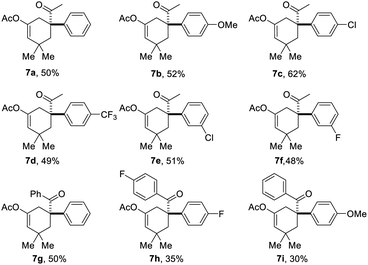
Based on the above results, the application of this method in the construction of a functionalized cyclohexane skeleton with a phenyl-substituted quaternary carbon center, a common moiety in a variety of natural products like limaspermidine15a and strychnine,15b was further investigated. Accordingly, a series of allylic bromides with an aryl substituted tertiary alcohol moiety were applied to this reaction with substrate 1a. All of the tested substrates afforded the expected products, and the regioselectivity of this reaction during the migration step agrees well with the common semipinacol rearrangement pattern, i.e., aryl groups and aryl groups with an electron-donating substituent are more preferred than alkyl groups and aryl groups with an electron-withdrawing substituent, respectively. For example, substrates 6a–6f all gave the aryl group migrated products, and substrate 6i led to ketone 7i as the sole product. Besides, the steric hindrance effect of the substituent on the aromatic ring has also been clearly observed. When the substituent on the phenyl ring was chloro, product 7c with the substituent at the para-position was obtained in higher yield than the one with it at the meta-position (7e).
Synthetic application
In order to demonstrate the efficiency of this method in constructing a highly complex structural skeleton, a quick assembly of the key tetracyclic skeleton of waihoensene,16 a unique diterpene molecule isolated from the New Zealand podocarp, featuring fused and strained tetracyclic rings and four consecutive congested all-carbon quaternary centers, was attempted using this reaction as the key step (Scheme 3). Due to the great difficulty in constructing such a tetracyclic framework with vicinal quaternary carbon centers, only one synthetic strategy toward waihoensene has been reported in 2017 using a tandem cycloaddition reaction of an allene substrate prepared in 12 steps as the key step.17 Herein, based on the method we developed, starting from prop-2-yn-1-yl acetate 1g and 2h synthesized from the known reagent 8 in 6 steps, a facile access to a tricyclic skeleton was accomplished affording 5p in 40% yield and a dr ratio of 3.2/1 using hexafluorobenzene as the solvent.18 The relative configuration of 5p and its diastereoisomer 5q was confirmed by the X-ray structure analysis of their derivatives.14 Next, the TBDPS protecting group of 5p was removed with TBAF followed by IBX oxidation providing dicarbonyl compound 12. A K2CO3-induced tandem hydrolysis/intramolecular aldol cyclization/elimination reaction would give compound 13. Thus a quick construction of the tetracyclic skeleton core of waihoensene with vicinal all-carbon centers was realized through the use of this key methodology. Additionally, an unprecedented [3.2.2] bridged motif 14 could be obtained through a different cyclization model in the presence of HCl.Conclusions
In summary, targeting a highly functionalized spirocyclo[4.5]decane skeleton, a purification free tandem Castro–Stephens coupling/1,3-acyloxy shift/cyclization/semipinacol rearrangement reaction of propargyl esters with allylic bromide has been successfully developed. This method not only features a highly efficient chemical conversion into complex spirobicyclic compounds from simple readily available substrates, but also exhibits a wide substrate scope. Especially, compared with our previous work on semipinacol rearrangement related synthetic methodology, some characteristic functional groups that are necessary for the corresponding bioactive natural products, such as isopropyl4g,h and geminal methyl groups,4a can be readily installed. Moreover, the generation of a vinyl ester moiety by this transformation provides an important hinge for subsequent transformation enabling a quick construction of the key 6/5/5/5-fused tetracyclic skeleton of waihoensene. Further application of this method for the total synthesis of related bioactive natural products is ongoing in the same lab.Conflicts of interest
The authors declare no competing interests.Acknowledgements
We greatly thank the NSFC (No. 21502080, 21772071, 21871117, 21772076, 21702136, and 91956203), the ‘111’ Program and Central Universities program (lzujbky-2018-134) of MOE, the Major project (2018ZX09711001-005-002) of MOST, the STCSM (19JC1430100), and the Department of Education of Guangdong Province (2019KZDXM035) for their financial support.Notes and references
- (a) P. Saraswat, G. Jeyabalan, M. Z. Hassan, M. U. Rahman and N. K. Nyola, Review of synthesis and various biological activities of spiro heterocyclic compounds comprising oxindole and pyrrolidine moieties, Synth. Commun., 2016, 46, 1643–1664 CrossRef CAS; (b) Y. Ma and C. Fan, Progress in the synthesis of spirotryprostatin alkaloids, Chin. J. Org. Chem., 2016, 36, 2380–2396 CrossRef CAS; (c) Y. Zheng, C. M. Tice and S. B. Singh, The use of spirocyclic scaffolds in drug discovery, Bioorg. Med. Chem. Lett., 2014, 24, 3673–3682 CrossRef CAS PubMed; (d) K. Ding, Z. Han and Z. Wang, Spiro skeletons: a class of privileged structure for chiral ligand design, Chem.–Asian J., 2009, 4, 32–41 CrossRef CAS PubMed.
- (a) S. Zhuo, T. Zhu, L. Zhou, C. Mou, H. Chai, Y. Lu, L. Pan, Z. Jin and Y. R. Chi, Access to all-carbon spirocycles through a carbene and thiourea cocatalytic desymmetrization cascade reaction, Angew. Chem., Int. Ed., 2019, 58, 1784–1788 CrossRef CAS PubMed; (b) Y. Li and S. Xu, Transition-metal-catalyzed C–H functionalization for construction of quaternary carbon centers, Chem.–Eur. J., 2018, 24, 16218–16245 CrossRef CAS PubMed; (c) S. Li, J. W. Zhang, X. L. Li, D. J. Cheng and B. Tan, Phosphoric acid-catalyzed asymmetric synthesis of SPINOL derivatives, J. Am. Chem. Soc., 2016, 138, 16561–16566 CrossRef CAS PubMed; (d) B. F. Rahemtulla, H. F. Clark and M. D. Smith, Catalytic enantioselective synthesis of C1- and C2-symmetric spirobiindanones through counterion-directed enolate C-acylation, Angew. Chem., Int. Ed., 2016, 55, 13180–13183 CrossRef CAS PubMed; (e) K. W. Quasdorf and L. E. Overman, Catalytic enantioselective synthesis of quaternary carbon stereocentres, Nature, 2014, 516, 181–191 CrossRef CAS PubMed; (f) A. Y. Hong and B. M. Stoltz, The construction of all-carbon quaternary stereocenters by use of Pd-catalyzed asymmetric allylic alkylation reactions in total synthesis, Eur. J. Org. Chem., 2013, 2745–2759 CrossRef CAS PubMed; (g) D. E. White, I. C. Stewart, R. H. Grubbs and B. M. Stoltz, The catalytic asymmetric total synthesis of elatol, J. Am. Chem. Soc., 2008, 130, 810–811 CrossRef CAS PubMed.
- For selected reviews: (a) P.-W. Xu, J.-S. Yu, C. Chen, Z.-Y. Cao, F. Zhou and J. Zhou, Catalytic enantioselective construction of spiro quaternary carbon stereocenters, ACS Catal., 2019, 9, 1820–1882 CrossRef CAS; (b) A. Ding, M. Meazza, H. Guo, J. W. Yang and R. Rios, New development in the enantioselective synthesis of spiro compounds, Chem. Soc. Rev., 2018, 47, 5946–5996 RSC; (c) S. Kotha, N. R. Panguluri and R. Ali, Design and synthesis of spirocycles, Eur. J. Org. Chem., 2017, 5316–5342 CrossRef CAS; (d) L. K. Smith and I. R. Baxendale, Total syntheses of natural products containing spirocarbocycles, Org. Biomol. Chem., 2015, 13, 9907–9933 RSC; (e) A. D'Yakonov, V. O. A. Trapeznikova, A. de Meijere and U. M. Dzhemilev, Metal complex catalysis in the synthesis of spirocarbocycles, Chem. Rev., 2014, 114, 5775–5814 CrossRef PubMed; (f) K. Undheim, Preparation and structure classification of heteroaspiro [m.n] alkanes, Synthesis, 2014, 46, 1957–2006 CrossRef CAS; (g) R. Rios, Enantioselective methodologies for the synthesis of spiro compounds, Chem. Soc. Rev., 2012, 41, 1060–1074 RSC; (h) S. Kotha, A. Deb, K. Lahiri and E. Manivannan, Selected synthetic strategies to spirocyclics, Synthesis, 2009, 165–193 CrossRef CAS.
-
(a) A. D. Rodríguez, C. Ramírez, I. I. Rodríguez and C. L. Barnes, Novel terpenoids from the West Indian sea whip Pseudopterogorgia elisabethae (Bayer). Elisapterosins A and B:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Rearranged diterpenes possessing an unprecedented
cagelike framework, J. Org. Chem., 2000, 65, 1390–1398 CrossRef PubMed;
(b) D. B. Clarke, S. F. R. Hinkley and R. T. Weavers, Waihoensene. A new laurenene-related diterpene from Podocarpus totara var waihoensis, Tetrahedron Lett., 1997, 38, 4297–4300 CrossRef CAS;
(c) Y. Fukuyama, N. Shida, M. Kodama, H. Chaki and T. Yugami, Tticycloillicinone, a novel prenylated C6–C3 compound increasing choline acetyltransferase (ChAT) activity, isolated from Illicium tashiroi, Chem. Pharm. Bull., 1995, 43, 2270–2272 CrossRef CAS PubMed;
(d) C. Pathirana, W. Fenical, E. Corcoran and J. Clardy, Erythrodiene: a new spirobicyclic sesquiterpene of a rare skeletal class from the caribbean gorgonian coral Erythropodium caribaeorum, Tetrahedron Lett., 1993, 34, 3371–3372 CrossRef CAS;
(e) A. Bagchi, Y. Oshima and H. Hikino, Spirojatamol, a new skeletal sesquiterpenoid of Nardostachys jatamansi roots, Tetrahedron, 1990, 46, 1523–1530 CrossRef CAS;
(f) P. Quitt, E. Mosettig, R. C. Cambie, P. S. Rutledge and L. H. Briggs, Optical rotatory dispersion studies. LVIII. 1. The complete absolute configurations of steviol, kaurene and the diterpene alkaloids of the garryfoline and atisine groups, J. Am. Chem. Soc., 1961, 83, 3720–3722 CrossRef;
(g) G. Stork and R. Breslow, The structure of cedrene, J. Am. Chem. Soc., 1953, 75, 3291 CrossRef CAS;
(h) P. Walter, Ueber das feste und flüssige Cedernöl, Ann. Chem., 1841, 39, 247–251 CrossRef.
Rearranged diterpenes possessing an unprecedented
cagelike framework, J. Org. Chem., 2000, 65, 1390–1398 CrossRef PubMed;
(b) D. B. Clarke, S. F. R. Hinkley and R. T. Weavers, Waihoensene. A new laurenene-related diterpene from Podocarpus totara var waihoensis, Tetrahedron Lett., 1997, 38, 4297–4300 CrossRef CAS;
(c) Y. Fukuyama, N. Shida, M. Kodama, H. Chaki and T. Yugami, Tticycloillicinone, a novel prenylated C6–C3 compound increasing choline acetyltransferase (ChAT) activity, isolated from Illicium tashiroi, Chem. Pharm. Bull., 1995, 43, 2270–2272 CrossRef CAS PubMed;
(d) C. Pathirana, W. Fenical, E. Corcoran and J. Clardy, Erythrodiene: a new spirobicyclic sesquiterpene of a rare skeletal class from the caribbean gorgonian coral Erythropodium caribaeorum, Tetrahedron Lett., 1993, 34, 3371–3372 CrossRef CAS;
(e) A. Bagchi, Y. Oshima and H. Hikino, Spirojatamol, a new skeletal sesquiterpenoid of Nardostachys jatamansi roots, Tetrahedron, 1990, 46, 1523–1530 CrossRef CAS;
(f) P. Quitt, E. Mosettig, R. C. Cambie, P. S. Rutledge and L. H. Briggs, Optical rotatory dispersion studies. LVIII. 1. The complete absolute configurations of steviol, kaurene and the diterpene alkaloids of the garryfoline and atisine groups, J. Am. Chem. Soc., 1961, 83, 3720–3722 CrossRef;
(g) G. Stork and R. Breslow, The structure of cedrene, J. Am. Chem. Soc., 1953, 75, 3291 CrossRef CAS;
(h) P. Walter, Ueber das feste und flüssige Cedernöl, Ann. Chem., 1841, 39, 247–251 CrossRef. - For selected examples of inter- and intramolecular cycloaddition reactions: (a) S. Poplata, A. Bauer, G. Storch and T. Bach, Intramolecular [2+2] photocycloaddition of cyclic enones: selectivity control by Lewis acids and mechanistic implications, Chem.–Eur. J., 2019, 25, 8135–8148 CrossRef CAS PubMed; (b) A. K. Clarke, J. T. Liddon, J. D. Cuthbertson, R. J. Taylor and W. P. Unsworth, Dearomatisation approaches to spirocyclic dienones via the electrophilic activation of alkynes, Org. Biomol. Chem., 2017, 15, 233–245 RSC; (c) B. S. Donslund, R. P. Nielsen, S. M. Mønsted and K. A. Jørgensen, Benzofulvenes in trienamine catalysis: stereoselective spiroindene synthesis, Angew. Chem., Int. Ed., 2016, 55, 11124–11128 CrossRef CAS PubMed; (d) K. Yoshida, Y. Itatsu, Y. Fujino, H. Inoue and K. Takao, Enantioselective organocatalytic construction of spiroindane derivatives by intramolecular Friedel–Crafts-type 1,4-addition, Angew. Chem., Int. Ed., 2016, 55, 6734–6738 CrossRef CAS PubMed; (e) J. Zheng, S.-B. Wang, C. Zheng and S.-L. You, Asymmetric dearomatization of naphthols via a Rh-catalyzed C(sp2)–H functionalization/annulation reaction, J. Am. Chem. Soc., 2015, 137, 4880–4883 CrossRef CAS PubMed; (f) L. Yang, H. Zheng, L. Luo, J. Nan, J. Liu, Y. Wang and X. Luan, Palladium-catalyzed dynamic kinetic asymmetric transformation of racemic biaryls: axial-to-central chirality transfer, J. Am. Chem. Soc., 2015, 137, 4876–4879 CrossRef CAS PubMed; (g) S. Rousseaux, J. García-Fortanet, M. A. Del Aguila Sanchez and S. L. Buchwald, Palladium(0)-catalyzed arylative dearomatization of phenols. Au-Containing all-carbon 1,3-dipoles: generation and [3+2] cycloaddition reactions, J. Am. Chem. Soc., 2011, 133, 9282–9285 CrossRef CAS PubMed.
- (a) X. M. Zhang, Y. Q. Tu, F. M. Zhang, Z. H. Chen and S. H. Wang, Recent applications of the 1,2-carbon atom migration strategy in complex natural product total synthesis, Chem. Soc. Rev., 2017, 46, 2272–2305 RSC; (b) S. H. Hou, Y. Q. Tu, S. H. Wang, C. C. Xi, F. M. Zhang, S. H. Wang, Y. T. Li and L. Liu, Total syntheses of the tetracyclic cyclopiane diterpenes conidiogenone, conidiogenol, and conidiogenone B, Angew. Chem., Int. Ed., 2016, 55, 4456–4460 CrossRef CAS PubMed; (c) S. H. Wang, B. S. Li and Y. Q. Tu, Catalytic asymmetric semipinacol rearrangements, Chem. Commun., 2014, 50, 2393–2408 RSC; (d) S. H. Wang, Y. Q. Tu and M. Tang, in: Comprehensive Organic Synthesis II, ed. G. A. Molander and P. Knochel, Pergamon, Oxford, 2014, vol. 3, pp. 795–852 Search PubMed; (e) Z. L. Song, C. A. Fan and Y. Q. Tu, Semipinacol rearrangement in natural product synthesis, Chem. Rev., 2011, 111, 7523–7556 CrossRef CAS PubMed; (f) B. Wang and Y. Q. Tu, Stereoselective construction of quaternary carbon stereocenters via a semipinacol rearrangement strategy, Acc. Chem. Res., 2011, 44, 1207–1222 CrossRef CAS PubMed; (g) E. Zhang, C. A. Fan, Y. Q. Tu, F. M. Zhang and Y. L. Song, Organocatalytic asymmetric vinylogous α-ketol rearrangement: enantioselective construction of chiral all-carbon quaternary stereocenters in spirocyclic diketones via semipinacol-type 1,2-carbon migration, J. Am. Chem. Soc., 2009, 131, 14626–14627 CrossRef CAS PubMed.
- For selected examples: (a) H. Wu, Q. Wang and J. Zhu, Copper-catalyzed enantioselective domino arylation/semipinacol rearrangement of allylic alcohols with diaryliodonium salts, Chem.–Eur. J., 2017, 23, 13037–13041 CrossRef CAS PubMed; (b) D. H. Lukamto and M. J. Gaunt, Enantioselective copper-catalyzed arylation-driven semipinacol rearrangement of tertiary allylic alcohols with diaryliodonium salts, J. Am. Chem. Soc., 2017, 139, 9160–9163 CrossRef CAS PubMed; (c) W. Li, X. Liu, F. Tan, X. Hao, J. Zheng, L. Lin and X. Feng, Catalytic asymmetric homologation of α-ketoesters with α-diazoesters: synthesis of succinate derivatives with chiral quaternary centers, Angew. Chem., Int. Ed., 2013, 52, 10883–10886 CrossRef CAS PubMed; (d) F. Romanov-Michailidis, L. Guenee and A. Alexakis, Enantioselective organocatalytic fluorination-induced Wagner–Meerwein rearrangement, Angew. Chem., Int. Ed., 2013, 52, 9266–9270 CrossRef CAS PubMed; (e) W. Li, X. Liu, X. Hao, Y. Cai, L. Lin and X. Feng, A catalytic asymmetric ring-expansion reaction of isatins and α-alkyl-α-diazoesters: highly efficient synthesis of functionalized 2-quinolone derivatives, Angew. Chem., Int. Ed., 2012, 51, 8644–8647 CrossRef CAS PubMed; (f) W. Li, J. Wang, X. Hu, K. Shen, W. Wang, Y. Chu, L. Lin, X. Liu and X. Feng, Catalytic asymmetric Roskamp reaction of α-alkyl-α-diazoesters with aromatic aldehydes: highly enantioselective synthesis of α-alkyl-β-keto esters, J. Am. Chem. Soc., 2010, 132, 8532–8533 CrossRef CAS PubMed; (g) H. S. Yeom, Y. Lee, J. Jeong, E. So, S. Hwang, J. E. Lee, S. S. Lee and S. Shin, Stereoselective one-pot synthesis of 1-aminoindanes and 5,6-fused azacycles using a gold-catalyzed redox-pinacol-Mannich–Michael cascade, Angew. Chem., Int. Ed., 2010, 49, 1611–1614 CrossRef CAS PubMed; (h) I. R. Pottie, S. N. Crane, A. L. Gosse, D. O. Miller and D. J. Burnell, Geminal acylation of-α-heterosubstituted cyclohexanones and their ketals, Can. J. Chem., 2010, 88, 1118–1124 CrossRef CAS; (i) X. Huang and L. Zhang, Two-step formal [3+2] cycloaddition of enones/enals and allenyl MOM ether: Gold-catalyzed highly diastereoselective synthesis of cyclopentanone enol ether containing an all-carbon quaternary center, J. Am. Chem. Soc., 2007, 129, 6398–6399 CrossRef CAS PubMed; (j) S. F. Kirsch, J. T. Binder, B. Crone, A. Duschek, T. T. Haug, C. Liebert and H. Menz, Catalyzed tandem reaction of 3-silyloxy-1,5-enynes consisting of cyclization and pinacol rearrangement, Angew. Chem., Int. Ed., 2007, 46, 2310–2313 CrossRef CAS PubMed; (k) G. R. Dake, M. D. B. Fenster, M. Fleury and B. O. Patrick, Investigations of α-siloxy-epoxide ring expansions forming 1-azaspirocyclic ketones, J. Org. Chem., 2004, 69, 5676–5683 CrossRef CAS PubMed.
- For selected examples of the Au-catalyzed 1,3-OAc shift of propargylic ester and cyclization:
(a) M. Brandstätter, N. Huwylera and E. M. Carreira, Gold(I)-catalyzed stereoselective cyclization of 1,3-enyne aldehydes by a 1,3-acyloxy migration/Nazarov cyclization/aldol addition cascade, Chem. Sci., 2019, 10, 8219–8223 RSC;
(b) S. K. Thummanapelli, S. Hosseyni, Y. Su, N. G. Akhmedov and X. Shi, Ligand-controlled gold-catalyzed cycloisomerization of 1,n-enyne esters toward synthesis of dihydronaphthalene, Chem. Commun., 2016, 52, 7687–7690 RSC;
(c) C. Chen, Y. Zou, X. Chen, X. Zhang, W. Rao and P. W. H. Chan, Gold-catalyzed tandem 1,3-migration/double cyclopropanation of 1-ene-4,n-diyne esters to tetracyclodecene and tetracycloundecene derivatives, Org. Lett., 2016, 18, 4730–4733 CrossRef CAS PubMed;
(d) Z. Cao and F. Gagosz, Gold-catalyzed tandem cycloisomerization/Cope rearrangement: an efficient access to the hydroazulenic motif, Angew. Chem., Int. Ed., 2013, 52, 9014–9018 CrossRef CAS PubMed;
(e) Y. Chen, M. Chen and Y. Liu, Gold-catalyzed cascade cyclizations of 1,6-diynyl carbonates to benzo[b]fluorenes involving arylation of oxocarbenium ion intermediates and decarboxylative etherification, Angew. Chem., Int. Ed., 2012, 51, 6493–6497 CrossRef CAS PubMed;
(f) D. Lebœuf, A. Simonneau, C. Aubert, M. Malacria, V. Gandon and L. Fensterbank, Gold-catalyzed 1,3-acyloxy migration/5-exo-dig cyclization/1,5-acyl migration of diynyl esters, Angew. Chem., Int. Ed., 2011, 50, 6868–6871 CrossRef PubMed;
(g) S. Bhunia and R.-S. Liu, Gold-catalyzed 1,3-addition of a sp3-hybridized C–H bond to alkenylcarbenoid intermediate, J. Am. Chem. Soc., 2008, 130, 16488–16489 CrossRef CAS PubMed;
(h) F.-Q. Shi, X. Li, Y. Xia, L. Zhang and Z.-X. Yu, DFT study of the mechanisms of in water Au(I)-catalyzed tandem [3,3]-rearrangement/Nazarov reaction/[1,2]-hydrogen shift of enynyl acetates:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a proton-transport catalysis strategy in the water-catalyzed [1,2]-hydrogen shift, J. Am. Chem. Soc., 2007, 129, 15503–15512 CrossRef CAS PubMed;
(i) A. Buzas and F. Gagosz, Gold(I) catalyzed isomerization of 5-en-2-yn-1-yl acetates: an efficient access to acetoxyl bicyclo[3.1.0]hexenes and 2-cycloalken-1-ones, J. Am. Chem. Soc., 2006, 128, 12614–12615 CrossRef CAS PubMed;
(j) J. Zhao, C. O. Hughes and F. D. Toste, Synthesis of aromatic ketones by a transition metal-catalyzed tandem sequence, J. Am. Chem. Soc., 2006, 128, 7436–7437 CrossRef CAS PubMed;
(k) L. Zhang and S. Wang, Efficient synthesis of cyclopentenones from enynyl acetates via tandem Au(I)-catalyzed 3,3-rearrangement and the Nazarov reaction, J. Am. Chem. Soc., 2006, 128, 1442–1443 CrossRef CAS PubMed.
a proton-transport catalysis strategy in the water-catalyzed [1,2]-hydrogen shift, J. Am. Chem. Soc., 2007, 129, 15503–15512 CrossRef CAS PubMed;
(i) A. Buzas and F. Gagosz, Gold(I) catalyzed isomerization of 5-en-2-yn-1-yl acetates: an efficient access to acetoxyl bicyclo[3.1.0]hexenes and 2-cycloalken-1-ones, J. Am. Chem. Soc., 2006, 128, 12614–12615 CrossRef CAS PubMed;
(j) J. Zhao, C. O. Hughes and F. D. Toste, Synthesis of aromatic ketones by a transition metal-catalyzed tandem sequence, J. Am. Chem. Soc., 2006, 128, 7436–7437 CrossRef CAS PubMed;
(k) L. Zhang and S. Wang, Efficient synthesis of cyclopentenones from enynyl acetates via tandem Au(I)-catalyzed 3,3-rearrangement and the Nazarov reaction, J. Am. Chem. Soc., 2006, 128, 1442–1443 CrossRef CAS PubMed. - For selected reviews: (a) D. Pflasterer and A. S. Hashmi, Gold catalysis in total synthesis – recent achievements, Chem. Soc. Rev., 2016, 45, 1331–1367 RSC; (b) Y. Zhu, L. Sun, P. Lu and Y. Wang, Recent advances on the Lewis acid-catalyzed cascade rearrangements of propargylic alcohols and their derivatives, ACS Catal., 2014, 4, 1911–1925 CrossRef CAS; (c) R. Kazem Shiroodi and V. Gevorgyan, Metal-catalyzed double migratory cascade reactions of propargylic esters and phosphates, Chem. Soc. Rev., 2013, 42, 4991–5001 RSC; (d) N. Krause and C. Winter, Gold-catalyzed nucleophilic cyclization of functionalized allenes: a powerful access to carbo- and heterocycles, Chem. Rev., 2011, 111, 1994–2009 CrossRef CAS PubMed.
- (a) J. D. White, R. G. Carter, K. F. Sundermann and M. Wartmann, Total synthesis of epothilone B, epothilone D, and cis- and trans-9,10-dehydroepothilone D, J. Am. Chem. Soc., 2001, 123, 5407–5413 CrossRef CAS PubMed; (b) R. D. Stephens and C. E. Castro, The substitution of aryl iodides with cuprous acetylides. A synthesis of tolanes and heterocyclics, J. Org. Chem., 1963, 28, 3313–3315 CrossRef CAS.
-
(a) A. W. Sromek, A. V. Kel'in and V. Gevorgyan, A novel 1,2-migration of acyloxy, phosphatyloxy, and sulfonyloxy groups in allenes: efficient synthesis of tri- and tetrasubstituted furans, Angew. Chem., Int. Ed., 2004, 43, 2280–2282 CrossRef CAS PubMed;
(b) T. Schwier, A. W. Sromek, D. M. L. Yap, D. Chernyak and V. Gevorgyan, Mechanistically diverse copper-, silver-, and gold-catalyzed acyloxy and phosphatyloxy migrations:
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) efficient synthesis of heterocycles via cascade migration/cycloisomerization approach, J. Am. Chem. Soc., 2007, 129, 9868–9878 CrossRef CAS PubMed.
efficient synthesis of heterocycles via cascade migration/cycloisomerization approach, J. Am. Chem. Soc., 2007, 129, 9868–9878 CrossRef CAS PubMed. - For detailed optimization of tandem reaction conditions, see ESI.†.
- M. Jia and M. Bandini, Counterion effects in homogeneous gold catalysis, ACS Catal., 2015, 5, 1638–1652 CrossRef CAS.
- For the X-ray crystallography of corresponding derivatives, please see ESI.†.
- (a) J. D. Medina and L. D. Genova, Alkaloids of the bark of Aspidosperma rhombeosignatum, Planta Med., 1979, 37, 165–167 CrossRef CAS; (b) R. B. Woodward and W. J. Brehm, The structure of strychnine. Formulation of the neo bases, J. Am. Chem. Soc., 1948, 70, 2107–2115 CrossRef CAS PubMed.
- (a) S. Li, P. Zhang, Y. Li, S. Lu, J. Gong and Z. Yang, Diastereoselective synthesis of diquinanes and triquinanes bearing vicinal quaternary carbon stereocenters from acyclic allene-based precursors via a cascade reaction, Org. Lett., 2017, 19, 4416–4419 CrossRef CAS PubMed; (b) E. V. Prusov, Construction of quaternary stereogenic centers in the total synthesis of natural products, Angew. Chem., Int. Ed., 2017, 56, 14356–14358 CrossRef CAS PubMed; (c) J.-K. Erggden and H. W. Moore, A new tandem route to angular tetraquinanes. Synthesis of the waihoensene ring system, Org. Lett., 1999, 1, 375–377 CrossRef PubMed.
- H. Lee, T. Kang and H. Y. Lee, Total synthesis of (±)-waihoensene, Angew. Chem., Int. Ed., 2017, 56, 8254–8257 CrossRef CAS PubMed.
- For detailed optimization of reaction conditions, see ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 1962272, 1962275, 1962276, 1962295, 1962296 and 1962417. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/d0sc00102c |
| This journal is © The Royal Society of Chemistry 2020 |