Themed collection Amyloids and Protein Aggregation

Amyloids and protein aggregation
Sara Linse and Tuomas Knowles introduce the Chemical Science themed collection on the topic of amyloids and protein aggregation.

Chem. Sci., 2023,14, 6491-6492
https://doi.org/10.1039/D2SC90225G
The supersaturation perspective on the amyloid hypothesis
The current amyloid hypothesis does not capture the full complexity of Aβ aggregation. Here we lay out a supersaturation framework to better understand the molecular mechanism of Alzheimer’s disease and to develop more effective treatment strategies.

Chem. Sci., 2024,15, 46-54
https://doi.org/10.1039/D3SC03981A
Molecular mechanisms of amyloid formation in living systems
The molecular mechanisms of amyloid formation have been studied extensively in test tube reactions. This perspective article addresses the question to what extent these mechanisms apply to the complex situation in living cells and organisms.
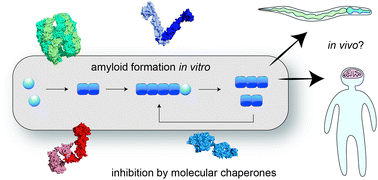
Chem. Sci., 2022,13, 7080-7097
https://doi.org/10.1039/D2SC01278B
Stability matters, too – the thermodynamics of amyloid fibril formation
The thermodynamics of amyloid formation has largely been neglected compared to kinetic studies. In this review, the current state of the experimental exploration of amyloid thermodynamics is presented and important open questions are highlighted.

Chem. Sci., 2022,13, 10177-10192
https://doi.org/10.1039/D1SC06782F
Functional amyloids from bacterial biofilms – structural properties and interaction partners
Functional bacterial amyloids forming biofilms have unique structural characteristics while still being similar to pathological ones. Through many identified interaction partners, they emerge as complex and essential components of biofilms.
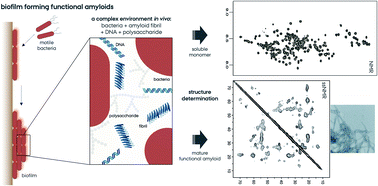
Chem. Sci., 2022,13, 6457-6477
https://doi.org/10.1039/D2SC00645F
Site specific NMR characterization of abeta-40 oligomers cross seeded by abeta-42 oligomers
Extracellular accumulation of β amyloid peptides of 40 (Aβ40) and 42 residues (Aβ42) has been considered as one of the hallmarks in the pathology of Alzheimer's disease.
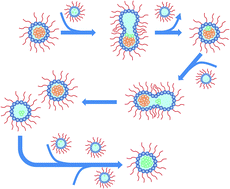
Chem. Sci., 2022,13, 8526-8535
https://doi.org/10.1039/D2SC01555B
Amyloid-β peptide 37, 38 and 40 individually and cooperatively inhibit amyloid-β 42 aggregation
The pathology of Alzheimer's disease is connected to the aggregation of β-amyloid (Aβ) peptide, which in vivo exists as a number of length-variants. This study identifies the Aβ37/38/40 ratio that is maximally inhibitory to Aβ42 aggregation.

Chem. Sci., 2022,13, 2423-2439
https://doi.org/10.1039/D1SC02990H
Computational maturation of a single-domain antibody against Aβ42 aggregation
A computational maturation method enables the generation of an antibody variant with over 200-fold increased potency against the primary nucleation process in Aβ42 aggregation.
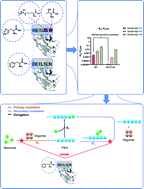
Chem. Sci., 2021,12, 13940-13948
https://doi.org/10.1039/D1SC03898B
Quantitative interrogation of protein co-aggregation using multi-color fluorogenic protein aggregation sensors
A little leak will sink a great ship! We prepared a series of multi-color protein aggregation sensors and developed a dual-color thermal shift assay to simultaneously and quantitatively report on protein co-aggregation of two different proteins.
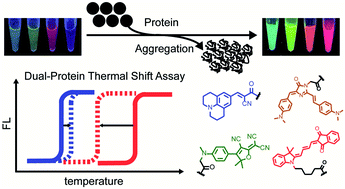
Chem. Sci., 2021,12, 8468-8476
https://doi.org/10.1039/D1SC01122G
3D-visualization of amyloid-β oligomer interactions with lipid membranes by cryo-electron tomography
Cryo-electron tomography 3D imaging of amyloid-β oligomers carpeting the surface of lipid bilayers in near native conditions.
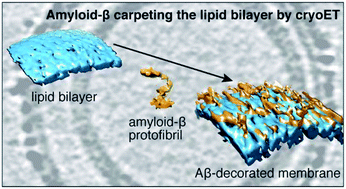
Chem. Sci., 2021,12, 6896-6907
https://doi.org/10.1039/D0SC06426B
Sequestration within biomolecular condensates inhibits Aβ-42 amyloid formation
Biomolecular condensates sequester an aggregation-prone peptide and prevent its aggregation, showing that heterotypic interactions within the condensates can prevent the formation of amyloid fibrils, despite the local increase in concentration.
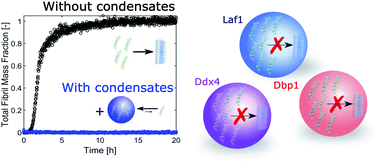
Chem. Sci., 2021,12, 4373-4382
https://doi.org/10.1039/D0SC04395H
The extent of protein hydration dictates the preference for heterogeneous or homogeneous nucleation generating either parallel or antiparallel β-sheet α-synuclein aggregates
The extent of protein hydration modulates the free energy barrier of both heterogeneous and homogeneous α-synuclein nucleation, leading to the formation of distinct amyloid polymorphs depending on the water activity of the protein microenvironment.
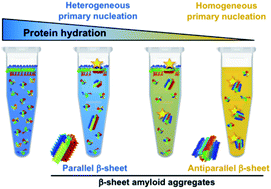
Chem. Sci., 2020,11, 11902-11914
https://doi.org/10.1039/D0SC05297C
Inhibitor and substrate cooperate to inhibit amyloid fibril elongation of α-synuclein
Amyloid fibril elongation of α-synuclein can be described with the Michaelis–Menten model, where α-synuclein monomer plays a dual role by serving as growth substrate as well as supporting the competitive inhibitor CC48 in blocking fibril ends.
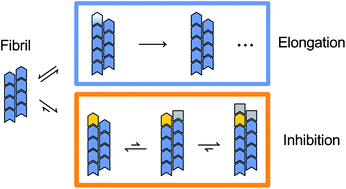
Chem. Sci., 2020,11, 11331-11337
https://doi.org/10.1039/D0SC04051G
Molecular chirality mediated amyloid formation on phospholipid surfaces
A remarkable inhibition effect and chiral discrimination are observed when the amyloid peptide aggregates on chiral phospholipid surfaces.
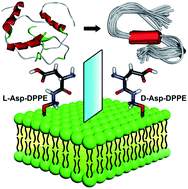
Chem. Sci., 2020,11, 7369-7378
https://doi.org/10.1039/D0SC02212H
Identification of on- and off-pathway oligomers in amyloid fibril formation
A general non-binary definition for on- and off-pathway intermediates is developed, enabling comparison of amyloid oligomers' contributions to fibril formation.
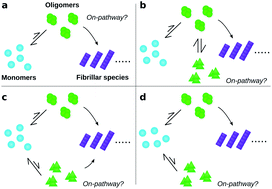
Chem. Sci., 2020,11, 6236-6247
https://doi.org/10.1039/C9SC06501F
Differentiating Aβ40 and Aβ42 in amyloid plaques with a small molecule fluorescence probe
A small molecule fluorescence probe ICTAD-1 was rationally designed for differentiating Aβ40 and Aβ42 in solutions and in Aβ plaques.
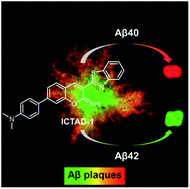
Chem. Sci., 2020,11, 5238-5245
https://doi.org/10.1039/D0SC02060E
Effects of sedimentation, microgravity, hydrodynamic mixing and air–water interface on α-synuclein amyloid formation
A comprehensive analysis on the impact of sedimentation, microgravity hydrodynamic mixing and air–water interface on α-synuclein aggregation kinetics.
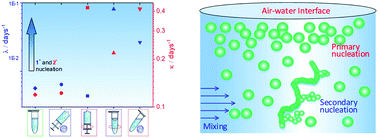
Chem. Sci., 2020,11, 3687-3693
https://doi.org/10.1039/D0SC00281J
Single molecule sensing of amyloid-β aggregation by confined glass nanopores
We have employed glass nanopore as a single molecule technique for direct sensing amyloidosis process of Aβ1–42 peptide, which of great significance in Alzheimer's disease.
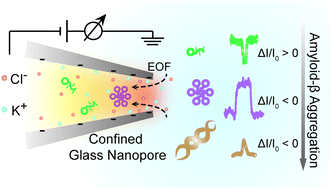
Chem. Sci., 2019,10, 10728-10732
https://doi.org/10.1039/C9SC03260F
Atomic resolution map of the soluble amyloid beta assembly toxic surfaces
Atomic resolution map of the soluble amyloid beta assembly (Aβn) “toxic surfaces” that facilitate the early pathogenic events in Alzheimer's disease (AD).
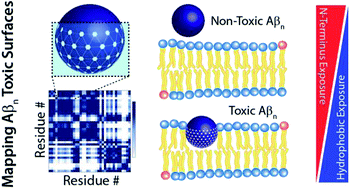
Chem. Sci., 2019,10, 6072-6082
https://doi.org/10.1039/C9SC01331H
Modular genetic design of multi-domain functional amyloids: insights into self-assembly and functional properties
Modular genetic design of functional amyloids represents new opportunities for creating multifunctional molecular materials with tailored structures and performance.

Chem. Sci., 2019,10, 4004-4014
https://doi.org/10.1039/C9SC00208A
Origin of metastable oligomers and their effects on amyloid fibril self-assembly
Simultaneous analysis of oligomer and fibril assembly kinetics reveals inhibitory effects of metastable oligomers on amyloid fibril formation.
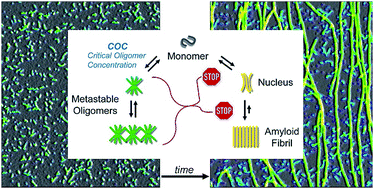
Chem. Sci., 2018,9, 5937-5948
https://doi.org/10.1039/C8SC01479E
C-terminal truncation of α-synuclein promotes amyloid fibril amplification at physiological pH
C-terminal truncations shift the pH range at which α-synuclein secondary nucleation occurs from acidic to neutral values.
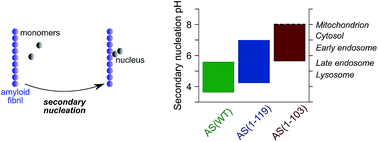
Chem. Sci., 2018,9, 5506-5516
https://doi.org/10.1039/C8SC01109E
Folding mechanisms steer the amyloid fibril formation propensity of highly homologous proteins
Understanding the molecular determinants of fibrillogenesis by studying the aggregation propensities of high homologous proteins with different folding pathways.
Chem. Sci., 2018,9, 3290-3298
https://doi.org/10.1039/C8SC00166A
Amyloid β-peptides 1–40 and 1–42 form oligomers with mixed β-sheets
Aβ40 and Aβ42 co-aggregate and form oligomers with mixed β-sheets as revealed by isotope-edited infrared spectroscopy.
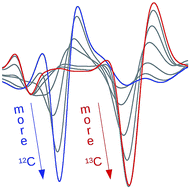
Chem. Sci., 2017,8, 8247-8254
https://doi.org/10.1039/C7SC01743J
Scaling behaviour and rate-determining steps in filamentous self-assembly
A general reaction network for filamentous self-assembly unifies mechanistic descriptions and links the overall scaling behaviour to the underlying rate-determining steps.
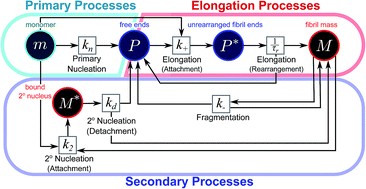
Chem. Sci., 2017,8, 7087-7097
https://doi.org/10.1039/C7SC01965C
Understanding co-polymerization in amyloid formation by direct observation of mixed oligomers
Co-assembly into hetero-oligomers controls the lag time of amylin assembly by a mechanism reminiscent of prions.
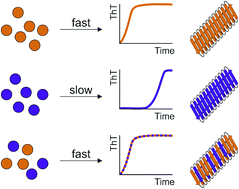
Chem. Sci., 2017,8, 5030-5040
https://doi.org/10.1039/C7SC00620A
Modulation of electrostatic interactions to reveal a reaction network unifying the aggregation behaviour of the Aβ42 peptide and its variants
The aggregation of Aβ42, linked to Alzheimer's disease, can be altered significantly by variations of the ionic strength of the solution, providing a means to relate the differences in aggregation mechanism of other Ab variants to changes in electrostatic interactions.
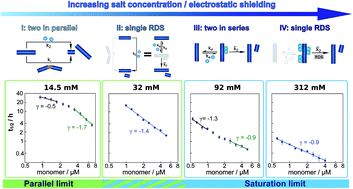
Chem. Sci., 2017,8, 4352-4362
https://doi.org/10.1039/C7SC00215G
The Aβ40 and Aβ42 peptides self-assemble into separate homomolecular fibrils in binary mixtures but cross-react during primary nucleation
Reaction network starting from monomer mixtures of Aβ40 and Aβ42. Interaction at the level of primary nucleation only accelerates Aβ40 fibril formation. Separate fibrils form as secondary nucleation and elongation are highly specific.

Chem. Sci., 2015,6, 4215-4233
https://doi.org/10.1039/C4SC02517B
About this collection
A number of proteins and peptides have a marked propensity to form amyloid fibrils, which are known as a hallmark of some of the most prevalent neurodegenerative diseases. Guest Editors, Sara Linse (Lund University, Sweden) and Tuomas Knowles (University of Cambridge, UK), introduce this themed collection on amyloids and protein aggregation, highlighting recent research and reviews in Chemical Science that look at key mechanistic findings in the process of amyloid formation, including thermodynamics, kinetics, oligomeric intermediates and the role of solution conditions.